This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Simulation model shows ribosome tunnel's impact on protein structures

Researchers at University of Tsukuba have developed a new model that simulates the internal environment of a ribosome—the cellular site of protein synthesis. Using computer simulations, they have analyzed the structures of various proteins within this model. Their findings reveal that the chemical properties of the ribosome tunnel, through which newly synthesized proteins pass, play crucial roles in the formation of protein structures during translation.
Proteins are synthesized by ribosomes and subsequently released through a tubular channel known as the ribosome tunnel. Recent studies suggest that some proteins start forming functionally important three-dimensional (3D) structures while still inside the ribosome tunnel. However, the exact mechanism underlying this process remains poorly understood.
To address these issues, researchers at the University of Tsukuba have comprehensively analyzed the 3D structures of ribosome tunnels reported to date. The study is published in the Journal of Chemical Information and Modeling.
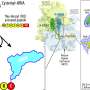
Based on this investigation, they developed a cylindrical model called the ribosome environment mimicking model (REMM), which replicates both the inner diameters and chemical properties of ribosome tunnels—key characteristics of the ribosome.
For comparison, they also fabricated a conventional carbon nanotube (CNT) model—a tubular molecule solely made of carbon atoms—which replicates only the inner diameter of the tunnel while disregarding its chemical properties.
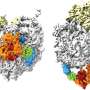
The team then used molecular dynamics simulations to examine protein structures within these models. Results revealed that the REMM more accurately reproduced the experimentally observed protein structures within the ribosome tunnel than the CNT.
Furthermore, chemical diversity in REMM was identified as a key factor contributing to their superior ability to replicate experimental protein structures. Further refinement of REMM is anticipated to enhance our understanding of protein conformations in living cells.
More information: Takunori Yasuda et al, Ribosome Tunnel Environment Drives the Formation of α-Helix during Cotranslational Folding, Journal of Chemical Information and Modeling (2024). DOI: 10.1021/acs.jcim.4c00901
Journal information: Journal of Chemical Information and Modeling
Provided by University of Tsukuba