This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study reveals new catalytic pathway for efficient water pollution control
Researchers have made a discovery in the field of water pollution control. They have developed a new technique using single-atom catalysts (SACs) within a Fenton-like catalytic system that significantly improves the efficiency of breaking down pollutants in water. Their findings have been published in the journal Nature Communications. The researchers are from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) and the Suzhou Institute for Advanced Study.
Single-atom catalysts are tiny, powerful tools in chemical reactions that can help clean water by breaking down harmful pollutants. However, their efficiency has been limited by the slow movement of reactants to the catalyst's surface and the high amount of oxidants needed. Previous research attributed efficiency improvements in nanoconfined SACs to the surface enrichment of pollutants and oxidants. However, the exact mechanisms were not fully understood.
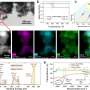
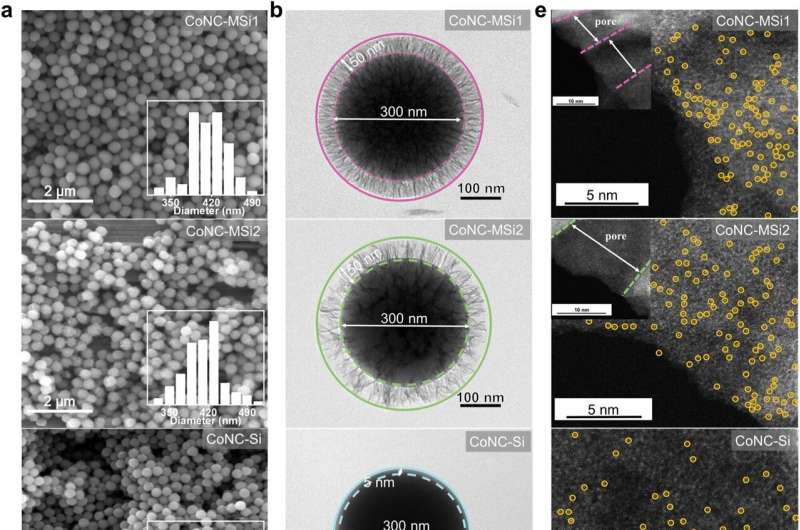
The research team discovered that by confining these catalysts within tiny, nanometer-sized pores in silica particles, they could dramatically speed up the reaction and use oxidants more efficiently. Their experiments showed that, apart from the local enrichment of reactants, the catalytic pathway itself changed. Instead of relying on singlet oxygen (a reactive form of oxygen), the reaction shifted to a direct electron transfer process, which is much more efficient for breaking down pollutants.
This new method resulted in an astonishing 34.7-fold increase in the rate of pollutant degradation compared to traditional methods. The efficiency of oxidant use also improved significantly, from 61.8% to 96.6%. The system proved to be highly effective in degrading various electron-rich phenolic compounds, demonstrating robustness in different environmental conditions and maintaining high performance in real lake water tests.
This research provides a deeper understanding of how nanoconfined catalysts work and opens up new possibilities for developing low-carbon, efficient water purification technologies. It offers a promising direction for further innovations in advanced oxidation processes and other applications in environmental science.
More information: Yan Meng et al, Nanoconfinement steers nonradical pathway transition in single atom fenton-like catalysis for improving oxidant utilization, Nature Communications (2024). DOI: 10.1038/s41467-024-49605-2
Journal information: Nature Communications
Provided by University of Science and Technology of China