This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemists succeed in upscaling a common reagent for industrial level applications

The metallic element samarium, when bound with other elements, is an incredibly useful chemical reagent for synthesizing molecules that can lead to new pharmaceuticals. Discovered in a Russian mine in 1879, the element was named after the mineral it was found in, called samarskite, which itself was named after Russian mining engineer Vassili Samarsky-Bykhovets.
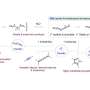
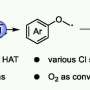
The most common samarium reagent is samarium diiodide, which consists of one atom of samarium and two atoms of the element iodine.
But scaling up this versatile reagent to quantities large enough to be used in industrial settings has proved challenging. "The reagent is air sensitive, so you often have to prepare the solution fresh, right before the reaction," says Caltech graduate student Chungkeun Shin, who works in the lab of Sarah Reisman, Bren Professor of Chemistry and the Norman Davidson Leadership Chair of Caltech's Division of Chemistry and Chemical Engineering.
"And we often have to use large amounts of it, even in small reactions, so it's not practical for running industrial-scale reactions."
As reported in the August 22 issue of the journal Science, Caltech chemists have succeeded in solving this scaling-up riddle. The study is titled "Reductive samarium (electro)catalysis enabled by SmIII-alkoxide protonolysis."
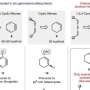
Their solution allows the samarium diiodide reagent to, essentially, recycle itself for repeated use in a single reaction, which means large amounts of solvents and fresh preparations are no longer needed.
"Samarium diiodide has been used in academia for the synthesis of natural products like taxol, an anticancer agent, but the reagent is not practical for creating products like this on industrial scales," says Reisman. "The breakthrough is that now we can translate some of these interesting reactions into process development or discovery."
The samarium reagent has been limited to lab use due to a pesky samarium–oxygen bond that forms during the reactions and renders the chemical inactive.
"It's been very difficult to recycle samarium back to its active state until now," explains Caltech graduate student Emily Boyd who works in the lab of Jonas Peters, Bren Professor of Chemistry and director of the Resnick Sustainability Institute at Caltech. Boyd and Shin are co-lead authors of the new study.
"The reagent often ends up with a very strong samarium-oxygen bond that is hard to break and makes it difficult to recycle the reagent," she says.
In other words, the oxygen bond leads to a dead-end for the reaction. "It's like the samarium reagent becomes lazy, sitting on a couch and doesn't want to do work," Shin says.
"It's very comfortable in this state and wants to stay that way," Boyd says. "So, we experimented with different acids to cleave the samarium-oxygen bond and get the reagent back to work."
Previous attempts to break this samarium–oxygen bond have required the use of harsh chemicals. In the new study, the researchers were able to cleave the bond using a mild acid, which is more practical for large-scale reactions. The acid supplies a proton to the bound oxygen, which turns it into an alcohol and frees up the samarium.
Boyd says that she and her colleagues in the Peters lab were interested in working with the Reisman lab because their research studies on nitrogen fixation involve the samarium diiodide agent.
Nitrogen fixation is the process by which gaseous nitrogen from our atmosphere is converted to compounds such as ammonia that are essential for plants (and the people who eat the plants). This process can be done naturally by bacteria, and artificially through chemical reactions. The Peters lab is developing new chemical reactions to artificially fix nitrogen in ways that are more efficient and sustainable than what is commonly used now.
"Taking nitrogen and converting it to ammonia is a reaction that our lab is deeply interested in," Boyd says. "We use the samarium reagent in our lab to study these reactions, but it would be impossible to scale this up to industrial levels. Through conversations with the Reisman group, which specializes in synthetic organic chemistry, we decided to join forces."
The collaboration proved synergistic. Shin explains, "I don't have the skills Emily has and vice versa. That combination allowed us to figure out the difficult chemistry."
More information: Emily A. Boyd et al, Reductive samarium (electro)catalysis enabled by SmIII -alkoxide protonolysis, Science (2024). DOI: 10.1126/science.adp5777
Journal information: Science
Provided by California Institute of Technology