May 3, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A fresh look at 1,2,3-cyclohexatriene shows it could be used as a versatile reagent in organic synthesis
A team of chemists at the University of California, Los Angeles has found that the benzene isomer 1,2,3-cyclohexatriene has the potential to be a versatile reagent in organic synthesis. In their study, reported in the journal Nature, Andrew Kelleghan, Ana Bulger, Dominick Witkowski and Neil Garg conducted experiments with the high-energy compound.
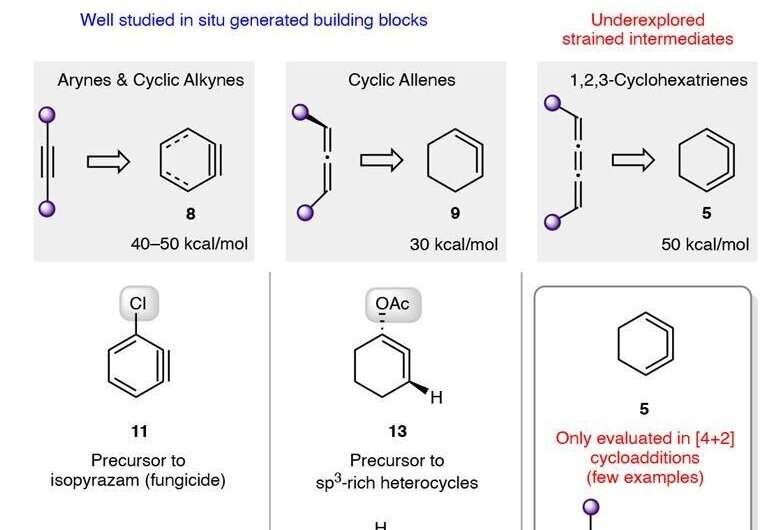
For many years, 1,2,3-cyclohexatriene has been virtually ignored as a useful reagent for use in organic synthesis—it has been considered to be too unstable to be of use. Still, it is promising because it is substantially higher in energy than benzene.
Also, isomers of benzene in general have long been of interest to organic chemists because of their potential for synthesizing useful organic materials. In this new effort, the researchers believed that under the right conditions, the isomer 1,2,3-cyclohexatriene could prove to be useful as a reagent for producing a wide range of organic compounds.
The team first set about producing samples using a method developed in the 1990s to generate intermediates from a precursor. They then used a variety of techniques to produce an array of fused-ring adducts, all of which were likely to react well, like benzenes, to nucleophiles. They then set to work using the resulting material to determine whether it could be used as an effective reagent.
To test the possibility of using it as electrophilic reagent, the team conducted a host of trapping reactions with dimethyl trienes. Then also used sily1 group members to show how nucleophilic reactions could be conducted and also conducted reactions showing the potential of σ-bond insertions. The group also conducted computational studies in conjunction with experimental analysis that showed that derivatives of 1,2,3-cyclohexatriene could be used in certain reactions despite the high degree of reactivity and its limited lifetime.
The team also showed that 1,2,3-cyclohexatrienes could be used in synthetic sequences that involve many steps, which, they note, shows their utility in building stereochemically and topologically complex molecules. They conclude that such isomers and their derivatives merit further investigation for use in creating organic materials.
More information: Andrew V. Kelleghan et al, Strain-promoted reactions of 1,2,3-cyclohexatriene and its derivatives, Nature (2023). DOI: 10.1038/s41586-023-06075-8
Journal information: Nature
© 2023 Science X Network