This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Highly selective catalyst enables 'one-step' conversion of methane to methanol
Scientists at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory and collaborating institutions have engineered a highly selective catalyst that can convert methane, a major component of natural gas, into methanol, an easily transportable liquid fuel, in a single, one-step reaction.
As described in a paper just published in the Journal of the American Chemical Society, this direct process for methane-to-methanol conversion runs at a temperature lower than required to make tea and exclusively produces methanol without additional byproducts.
That's a big advance over more complex traditional conversions that typically require three separate reactions, each under different conditions, including vastly higher temperatures.
"We pretty much throw everything into a pressure cooker, and then the reaction happens spontaneously," said chemical engineer Juan Jimenez, a Goldhaber postdoctoral fellow in Brookhaven Lab's Chemistry Division and the lead author on the paper.
The simplicity of the system could make it particularly useful for tapping "stranded" natural gas reserves in isolated rural areas, far from the costly infrastructure of pipelines and chemical refineries, said Brookhaven chemist and study co-author Sanjaya Senanayake. Such local deployments would remove the need to transport high-pressure, flammable liquified natural gas.
"We could scale up this technology and deploy it locally to produce methanol than can be used for fuel, electricity, and chemical production," Senanayake said.
Brookhaven Science Associates, which manages Brookhaven Lab on behalf of DOE, and the University of Udine, collaborators in this work, have filed a patent cooperation treaty application on the use of the catalyst for one-step methane conversion.
The team is exploring ways to work with entrepreneurial partners to bring the technology to market. They are motivated by the idea of "closing the carbon cycle"—essentially, recycling carbon to prevent it from being released into the atmosphere—to enable net-zero carbon clean-energy solutions.
"As scientists, we know the science and technology extremely well, but we're working with Brookhaven's Research Partnerships and Technology Transfer Office and entrepreneurial students who are doing the legwork on the economic side—figuring out who are the best potential clients and markets for expanding this out," Jimenez said.
From basic science to industry-ready
The basic science behind the conversion builds on a decade of collaborative research. The Brookhaven chemists worked with experts at the Lab's National Synchrotron Light Source II (NSLS-II) and Center for Functional Nanomaterials (CFN)—two DOE Office of Science user facilities that have a wide range of capabilities for tracking the intricacies of chemical reactions and the catalysts that enable them—as well as researchers at DOE's Ames National Laboratory and international collaborators in Italy and Spain.
Earlier studies worked with simpler ideal versions of the catalyst, consisting of metals on top of oxide supports or inverted oxide on metal materials. The scientists used computational modeling and a range of techniques at NSLS-II and CFN to learn how these catalysts work to break and remake chemical bonds to convert methane to methanol and to elucidate the role of water in the reaction.
"Those earlier studies were done on simplified model catalysts under very pristine conditions," Jimenez said. They gave the team valuable insights into what the catalysts should look like at the molecular scale and how the reaction would potentially proceed, "but they required translation to what a real-world catalytic material looks like," he said.
As Senanayake explained, "What Juan has done is take those concepts that we learned about the reaction and optimize them, working with our materials synthesis colleagues at the University of Udine in Italy, theorists at the Institute of Catalysis and Petrochemistry and Valencia Polytechnic University in Spain, and characterization colleagues here at Brookhaven and Ames Lab.
"This new work validates the ideas behind the earlier work and translates the lab-scale catalyst synthesis into a much more practical process for making kilogram-scale amounts of catalytic powder that are directly relevant to industrial applications."
New tools uncover the secret sauce
The new recipe for the catalyst contains an additional ingredient: a thin layer of "interfacial" carbon between the metal and oxide.
"Carbon is often overlooked as a catalyst," Jimenez said. "But in this study, we did a host of experiments and theoretical work that revealed that a fine layer of carbon between palladium and cerium oxide really drove the chemistry. It was pretty much the secret sauce. It helps the active metal, palladium, convert methane to methanol."
To explore and ultimately reveal this unique chemistry, the scientists built new research infrastructure both in the Catalysis Reactivity and Structure group's laboratory in the Chemistry Division and at NSLS-II.
"This is a three-phase reaction with gas, solid, and liquid ingredients—namely methane gas, hydrogen peroxide and water as liquids, and the solid powder catalyst—and these three ingredients react under pressure. So, we needed to build new pressurized three-phase reactors so we could monitor those ingredients in real time," Senanayake said.
The team built one reactor in the Chemistry Division and used infrared spectroscopy to measure the reaction rates and to identify the chemical species that arose on the catalyst surface as the reaction progressed. The chemists also relied on the expertise of NSLS-II scientists who built additional reactors to install at two NSLS-II beamlines—Inner-Shell Spectroscopy (ISS) and In situ and Operando Soft X-ray Spectroscopy (IOS)—so they could also study the reaction using X-ray techniques.
NSLS-II's Dominik Wierzbicki, a study co-author, worked to design the ISS reactor so the team could study the high-pressure, gas-solid-liquid reaction using X-ray spectroscopy. In this technique, "hard" X-rays, which have relatively high energies, enabled the scientists to follow the active metal, palladium, under realistic reaction conditions.
"Typically, this technique requires compromises because measuring the gas-liquid-solid interface is complex, and high pressure adds even more challenges," Wierzbicki said. "Adding unique capabilities to address these challenges at NSLS-II is advancing our mechanistic understanding of reactions carried out under high-pressure and opening new avenues for synchrotron research."
Study co-authors Iradwikanari Waluyo and Adrian Hunt, beamline scientists at IOS, also built an in-situ setup at their beamline and used it for lower energy "soft" X-ray spectroscopy to study cerium oxide in the gas-solid-liquid interface. These experiments revealed information about the nature of the active catalytic species during simulated reaction conditions.
"Correlating the information from the Chemistry Division to the two beamlines required synergy and is at the heart of the new capabilities," Senanayake said.
"This collaborative effort has yielded unique insights into how the reaction can occur," he added, noting this study as a first demonstration of how such multimodal characterization tools can advance scientists' understanding of high-pressure catalytic reactions.
"The tools we developed for this study now provide additional in situ capabilities for other NSLS-II users interested in studying chemistry under pressurized conditions at our beamlines," Waluyo said.
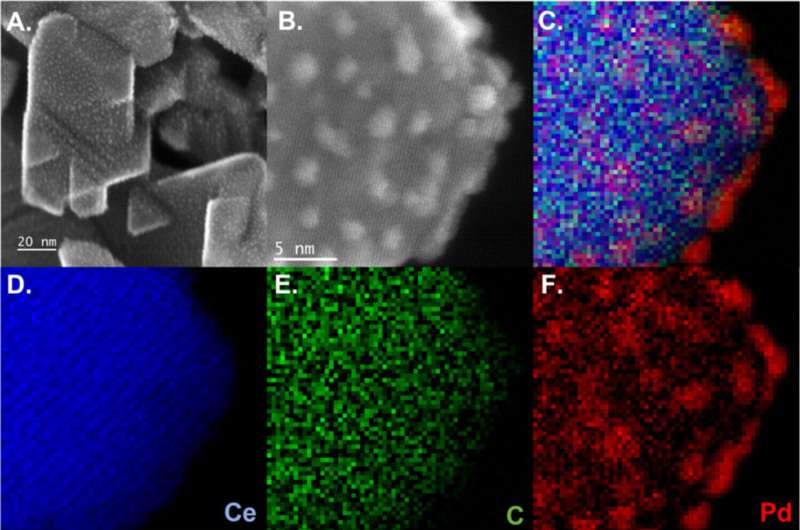
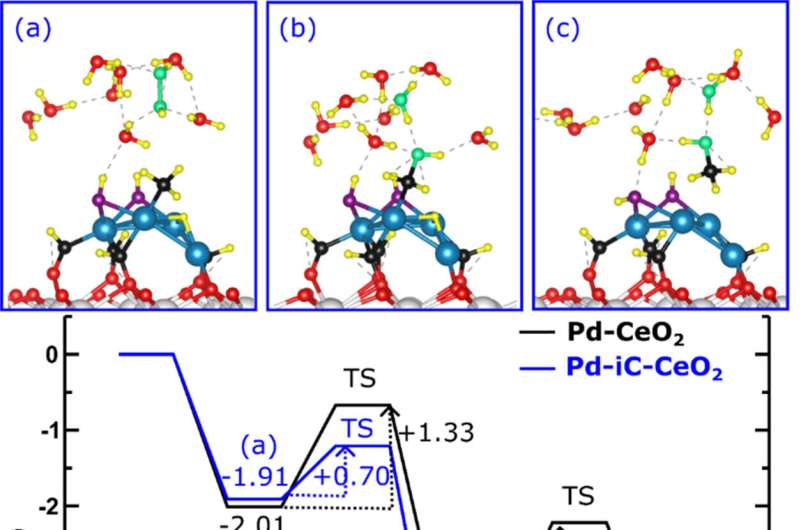
In addition, colleagues Jie Zhang and Long Qi at Ames Lab performed in situ nuclear magnetic resonance studies, which gave the scientists key insights into the early stages of the reaction; and Sooyeon Hwang at CFN produced stunning transmission electron microscopy images to identify the carbon present in the material. The team's theory colleagues in Spain, led by Verónica Ganduglia-Pirovano and Pablo Lustemberg, provided the theoretical explanation for the catalytic mechanism by developing a state-of-the-art computational model for the three-phase reaction.
"We worked with a global team to gain a comprehensive understanding of the reaction and mechanism," Senanayake said.
In the end, the team discovered how the active state of their three-component catalyst—made of palladium, cerium oxide, and carbon—exploits the complex three-phase, liquid-solid-gas microenvironment to produce the final product.
Now, instead of needing three separate reactions in three different reactors operating under three different sets of conditions to produce methanol from methane with the potential of byproducts that require costly separation steps, the team has a three-part catalyst that drives a three-phase reaction all in one reactor with 100% selectivity for methanol production.
"This is a very valuable example of carbon neutral processing," Senanayake said. "We look forward to seeing this technology deployed at scale to make use of currently untapped sources of methane."
John Gordon, chair of the Chemistry Division, stated, "This research is a demonstration of how innovations in catalyst design and a foundational understanding of how reactions occur can help advance chemical processes of the future."
More information: Juan D. Jiménez et al, From Methane to Methanol: Pd-iC-CeO2 Catalysts Engineered for High Selectivity via Mechanochemical Synthesis, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c04815
Journal information: Journal of the American Chemical Society
Provided by Brookhaven National Laboratory