This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New models help predict protein dynamic signatures
To the average person, knowing how a protein wiggles might not seem that exciting or pertinent, but then again, most people aren't fascinated by the natural movements and fluctuations of proteins and their functional properties. If, however, you were interested in designing new drugs, better understanding how diseases can be eradicated or enhancing biotechnology for industrial and therapeutic applications, you might be on the edge of your seat waiting to see what a new study on protein sequencing and crystallization has to offer.
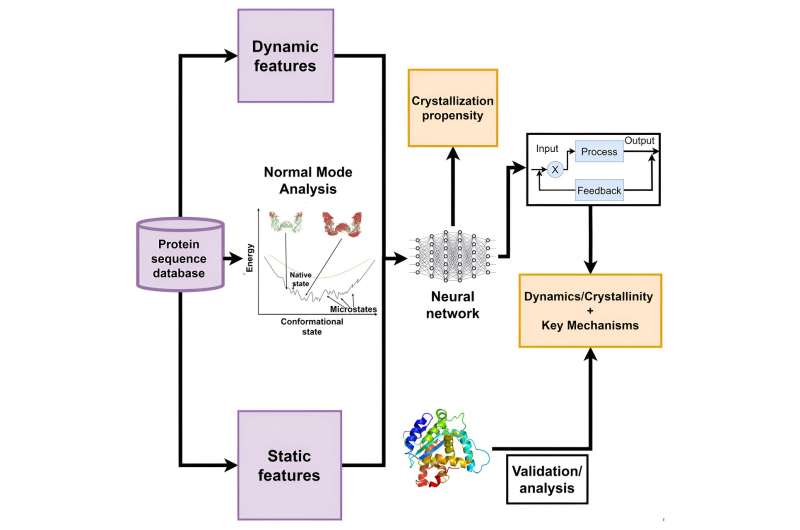
An article about that study, authored by Anna Tarakanova, assistant professor in the School of Mechanical, Aerospace, and Manufacturing Engineering at UConn's College of Engineering, has just appeared in the journal, Matter. The study examines how the natural movements and fluctuations of proteins—the protein's "wiggles"—can help predict their functional properties. Tarakanova was assisted by Mohammad Madani, a Mechanical Engineering graduate student and first author of the study.
In particular, Tarakanova and Madani focused on predicting the ability or propensity of proteins to form high-quality crystals. Protein crystallography is an important technique for understanding protein structures, which in turn is vital for developing drugs and understanding diseases. They developed a new computational model and tool that uses advanced techniques to analyze protein dynamics and predict their crystallization propensity accurately.
This tool, Tarakanova explains, can facilitate rational design of protein sequences that lead to diffraction-quality crystals. And their research showcases integration of physics-based and machine learning models for structure and property prediction, expanding the classical paradigm of structural biology.
"Our study shows that knowing what a protein is composed of (its amino acid sequence) and its structural features (or folds) is not always enough to predict how a protein will behave," Tarakanova says. "How a protein wiggles or fluctuates governs what it may be able to do. The model we developed is a framework that can be broadly extended to predicting protein functions by learning from the protein's dynamic signatures."
This research, she adds, represents a significant advancement in the field of structural biology. It integrates physical models that capture protein dynamics into crystallization prediction, which was not done comprehensively before. The new model developed, called DSDCrystal, outperforms existing models, making it a breakthrough in accurately predicting protein crystallization propensity.
Next steps, according to Tarakanova, will involve extending applications to broaden the utility of the model beyond crystallization propensity to other important protein properties.
"We can adapt the model to predict protein stability, which is important for understanding how proteins function under different conditions," she stresses.
"We'll also look at protein-to-protein interactions, modifying the model to predict interactions between different proteins, aiding in the study of complex biological processes. Additionally, we'll use the model to identify critical functional sites within proteins that are important for their biological activity."
The bottom line, Tarakanova states, is further developing a versatile tool that can predict multiple protein properties, thereby accelerating research in various fields of molecular biology. And while those kinds of predictions may not keep everyone up at night, for biotech researchers and scientists, understanding wiggling might be the way to untold future discoveries.
More information: Mohammad Madani et al, Protein dynamics inform protein structure: An interdisciplinary investigation of protein crystallization propensity, Matter (2024). DOI: 10.1016/j.matt.2024.04.023
Journal information: Matter
Provided by University of Connecticut