This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
The secret to sleepy cells' control of inflammatory secretions
Scientists at Sanford Burnham Prebys and the La Jolla Institute for Immunology have revealed a new secret regarding senescence, a cellular state similar to sleep that is more likely to affect aged cells. This drowsy condition is known to provide health benefits under certain conditions while also potentially causing collateral damage.
"Senescence is not all bad," said Peter D. Adams, Ph.D., the director of the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys and senior author on the new study. "It is a tumor suppression mechanism that prevents cancer by blocking the proliferation of potentially cancerous cells."
"It is also involved in orchestrating the wound healing response," added Nirmalya Dasgupta, Ph.D., instructor at the La Jolla Institute for Immunology, former postdoctoral associate in Adams' lab and first author on the study. "Through its inflammatory function, it can control tissue repair and wound healing."
However, senescence can be a double-edged sword with age and the immune system's declining efficiency at removing senescent cells. As these sleepy cells accumulate to unhealthy levels, they can stop aging tissues from properly regenerating.
"In addition to no longer growing and proliferating, the other hallmark of senescent cells is that they have this inflammatory program causing them to secrete inflammatory molecules," said Adams.
Too many of these secreted molecules can contribute to chronic inflammation in the body. This pervasive inflammation—called "inflammaging"—has been linked to many age-related diseases, such as rheumatoid arthritis, liver disease, atherosclerosis, muscle wasting (sarcopenia) and cancer.
Adams, Dasgupta and their collaborators have published results in Molecular Cell, describing a new connection between the inflammation caused by senescent cells and a protein involved in the process of winding up six feet of DNA tightly enough to fit into the nuclear center of cells.
The scientists defined how this protein influences the increase in inflammation when our cells slip into a state of slumber. By detailing this process, the authors may have uncovered a new opportunity to find drugs that can promote healthy aging by preventing or reducing chronic inflammation from the collection of too many senescent cells as we get older.
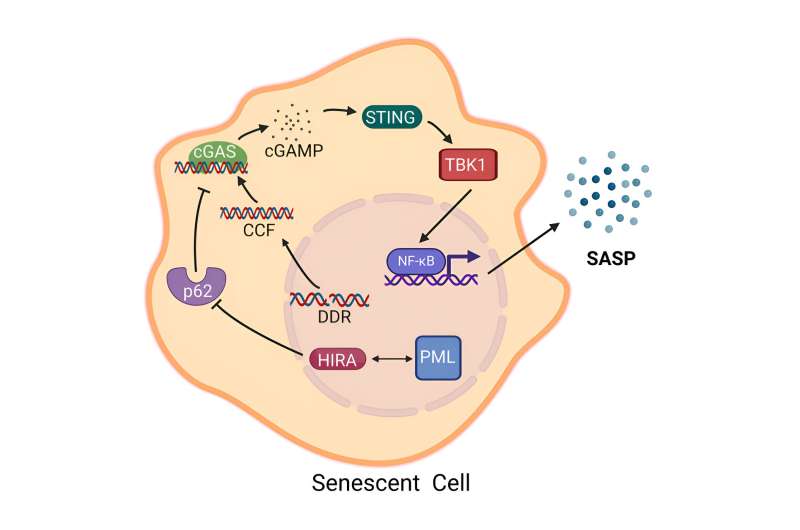
The research team began by modifying cells to deactivate the gene holding the codes for the HIRA protein, one of the histone chaperones responsible for helping to build spools out of histones used to hold DNA like a thread.
They also similarly silenced the gene for promyelocytic leukemia (PML) protein, which—when contained in tiny, dense spheres called nuclear bodies—serves as an anchor and organizing point for many proteins involved in a wide variety of functions, including the replication of complete copies of DNA during cell growth and the transcription of DNA into RNA during the building of new proteins. The scientists then forced the cells to become senescent and compared the modified cells to normal senescent cells.
The absence of HIRA and PML in the modified cells did not reverse the sleepy cells' lack of growth and proliferation. The two proteins did prove to be necessary for the cells to begin emitting the inflammatory molecules that can lead to inflammaging, which is known as senescence-associated secretory phenotype (SASP).
"One school of thought is that we should remove senescent cells to promote healthier aging," said Dasgupta. "An alternative view is that senescence has had a role throughout evolution, so removing it could be harmful. Under this view, reducing the inflammation from SASP may be more helpful and less risky, so learning more about its causes is crucial."
The research team followed up with additional experiments that demonstrated that HIRA had to move to PML nuclear bodies for senescent cells to enter their inflammatory state. The scientists also found that HIRA was essential for activating the pathway of cellular signals considered to be the primary way senescent cells begin oozing out inflammatory molecules.
In addition, the team discovered a new behavior for HIRA within the gel-like cytoplasm that occupies the space in the cell between the membrane and nucleus. HIRA physically interacts with a protein called p62 that was found to reduce the secretion of inflammatory molecules.
"With these results, we've defined a new pathway and new players in the process that kick-starts senescent cells' inflammatory program," said Adams. "This knowledge provides more opportunities to try and find new drugs to inhibit that process."
Adams says that the team will continue this research by collaborating with the team at Sanford Burnham Prebys' Conrad Prebys Center for Chemical Genomics (Prebys Center) to identify small molecules which target the newly defined pathway. The Prebys Center is a comprehensive center for drug discovery and chemical biology.
In addition to finding new drugs, it may be possible to repurpose existing drugs. Dasgupta said at least four drugs currently in cancer clinical trials may be effective at preventing HIRA from being transported to PML nuclear bodies. This movement by HIRA was found to be needed for senescent cells to secrete inflammatory molecules, marking drugs that can halt HIRA's localization to PML nuclear bodies as candidates for future research into healthy aging treatments.
"Our results also will help accelerate the work of the SenNet Consortium as we continue developing a detailed map of where these senescent cells are and what they look like," notes Adams.
Adams co-directs the San Diego Tissue Mapping Center within the Cellular Senescence Network (SenNet) Consortium, a large network of U.S. labs and research institutions.
"With our partners in the consortium, we want to know what senescent cells look like at the molecular level," said Adams. "We're looking for the gene expression programs and the signaling pathways which are activated in these cells, in different cell types and different tissues. With Nirmalya and the team, now we've uncovered another piece to the larger puzzle we're pulling together. Building a comprehensive map of senescence will enable us to better target senescent cells with treatments to promote healthy aging."
More information: Nirmalya Dasgupta et al, Histone chaperone HIRA, Promyelocytic Leukemia (PML) protein and p62/SQSTM1 coordinate to regulate inflammation during cell senescence., Molecular Cell (2024). DOI: 10.1016/j.molcel.2024.08.006. www.cell.com/molecular-cell/fu … 1097-2765(24)00660-9
Journal information: Molecular Cell
Provided by Sanford-Burnham Prebys