Direct oxidative transfer process contributes to water purification
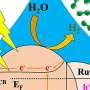
![Product analyses of the model reaction system (Co3O4/PMS/PhOH). a STEM, HAADF, and EDS elemental mapping images of the Co3O4 after the reaction. b TGA curves of the pristine and reacted Co3O4 in air (O2). The mass loss of 20% for the reacted Co3O4 was equal to the initial concentration ratio of [PhOH] to [PhOH] + [Co3O4], which indicates that the pollutant molecules were fully transferred to the catalyst surface. c 3D-FTIR spectra of the gas products detected from TGA of the reacted Co3O4 in b. The decomposition temperature (centered Around 300 °C) in air (O2) and the gas product (CO2) are characteristic of polymers. d, e XPS spectra (d) and FTIR spectra (e) of the pristine and reacted Co3O4. The signal intensities in the XPS spectra of the pristine and reacted Co3O4 were normalized by that of Co2p. Credit: Nature Communications (2022). DOI: 10.1038/s41467-022-30560-9 Direct oxidative transfer process contributes to water purification](https://scx1.b-cdn.net/csz/news/800a/2022/direct-oxidative-trans.jpg)
A research team led by Prof. Yu Hanqing from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences, collaborating with Prof. Menachem Elimelech from Yale University, developed a new water decontamination technology, the direct oxidative transfer process (DOTP). The study was published in Nature Communications.
Previous investigations showed that the removal of organic pollutants from water depends on an advanced oxidation process (AOP), which requires external energy or chemical input. However, it was discovered that the electron equivalent released by the pollutants was much higher than the oxidant-consumed electron equivalent, which could not be explained by AOP.
Researchers clarified that DOTP, fundamentally different from AOP, dominated the heterogeneous oxidative system. In DOPT, a direct redox reaction between pollutants and oxidants occurred on the catalyst surface. Products formed were stabilized and spontaneously underwent surface polymerization or coupling reaction. As a result, products accumulated on the catalyst surface, contributing to the effective elimination of aquatic pollutants.
The study reveals that the heterogeneous catalyst plays an important role in the activation, stabilization and accumulation of reactants or products. Additionally, it features low oxidant consumption, high pollutant accumulating capacity, and zero toxic byproducts. Thus, DOTP is expected to find further applications in water pollution control and wastewater treatment.
More information: Ying-Jie Zhang et al, Simultaneous nanocatalytic surface activation of pollutants and oxidants for highly efficient water decontamination, Nature Communications (2022). DOI: 10.1038/s41467-022-30560-9
Journal information: Nature Communications
Provided by Chinese Academy of Sciences