This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Mechanistic insights on SSZ-13 zeolite in catalytic dimethyl ether carbonylation
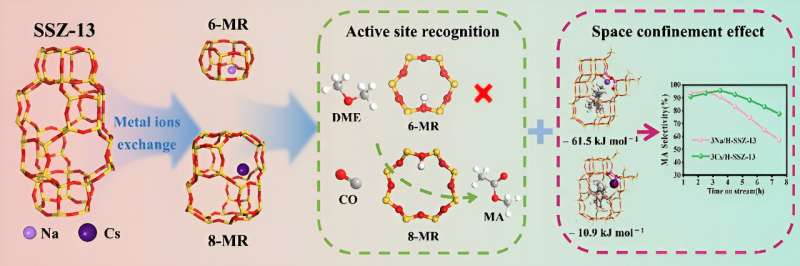
Dimethyl ether (DME) carbonylation to methyl acetate (MA) is one of the crucial steps in an indirect synthesis route of ethanol from syngas. SSZ-13 zeolite, with a typical topology of CHA, characterized by its 8-membered ring (8-MR) channels, has shown potential in catalyzing DME carbonylation. However, current studies lack a comprehensive understanding of the catalytic mechanism. Meanwhile, the influence of spatial confinement in SSZ-13 on the reaction has also not been considered.
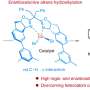
Recently, a research team led by Prof. Shouying Huang from Tianjin University, China, prepared a range of SSZ-13 samples with different metal loadings by simple ion exchange to fill this gap. Selective shielding of BAS within different channels of SSZ-13 is achieved by loading different sizes of Na+ and Cs+ metals.
The results were published in the Chinese Journal of Catalysis.
The characterization of acidity and Density Functional Theory (DFT) calculations confirm that at lower loading, Na+ preferentially settles in the 6-MR of SSZ-13 and replaces the BAS therein, while Cs+ can only interact with BAS in the 8-MR due to its large ionic radius. Combining activity data and the dissociation energies of reactants on BAS within different channels, it was indicated that both the main and side reactions of DME carbonylation occur on the 8-MR BAS of SSZ-13.
To highlight the role of spatial confinement in reaction, this work compared the pore structure of samples loaded with different sized metals, TG/DTG and GC-MS analyses of spent samples, and the adsorption stability of key intermediates in side reactions, proving that the SSZ-13 cage space is a key factor for affecting MA selectivity. Through metal loading to narrow the space of 8-MR, the formation of side-reaction intermediates can be efficiently inhibited, resulting in an improvement of the main reaction selectivity.
More information: Xiaomin Zhang et al, Mechanistic insights and the role of spatial confinement in catalytic dimethyl ether carbonylation over SSZ-13 zeolite, Chinese Journal of Catalysis (2024). DOI: 10.1016/S1872-2067(24)60040-9
Provided by Chinese Academy of Sciences