Void-confinement effect of nanoreactor promotes heterogeneous catalysis

A research team from the Hefei Institutes of Physical Science (HFIPS) of the Chinese Academy of Sciences has recently obtained a catalyst composed of the hollow carbon sphere and inner Ni nanoparticles (Ni@HCS), which displayed good performance when applied in the aqueous phase hydrogenation rearrangement tandem reaction of furfural (FAL), a typical aqueous phase reaction.
They found that it can afford 100 percent FAL conversion and 99 percent cyclopentanone selectivity at a mild condition (150 degrees Celsius, 2 MPa H2, 4h).
In heterogeneous catalysis, the deactivation of catalysts caused by active metal loss, agglomeration, and sintering has always been the crux of limiting the development of supported metal catalysts, especially in the aqueous phase under high temperature. In addition, improving the selectivity of the target product in the reaction is also a big challenge in catalyst preparation.
Thanks to the shape-selective catalysis induced by the void-confinement effect of the hollow structure, the selectivity of Ni@HCS to the target product is improved compared with active carbon supported catalysts.
More importantly, this hollow structure catalyst showed much better stability compared with active carbon supported catalysts as it can maintain its activity after ten cycle experiments. According to Chen Chun, a member of the team, it's because the protective effect of hollow carbon spheres on active metals greatly reduced the loss of metals in the reaction process.
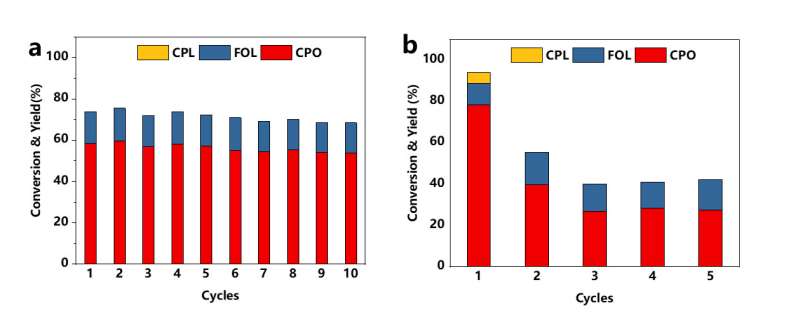
They have conducted more experiments to explained the phenomena. Inductively coupled plasma-atomic emission spectrometry results show that the Ni content of Ni@HCS has a tiny change after the reaction while the active carbon supported catalysts show over 60 percent metal loss during the reaction. In addition, the synthetic method is also applicable to other metal catalysts and other reactions.
This work has been published in Applied Catalysis B: Environmental.
More information: Zhi Hu et al, Hollow carbon sphere encapsulated nickel nanoreactor for aqueous-phase hydrogenation-rearrangement tandem reaction with enhanced catalytic performance, Applied Catalysis B: Environmental (2022). DOI: 10.1016/j.apcatb.2022.121140
Journal information: Applied Catalysis B: Environmental
Provided by Chinese Academy of Sciences