This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Research team overcomes heteroatom constraints via cobalt catalysis
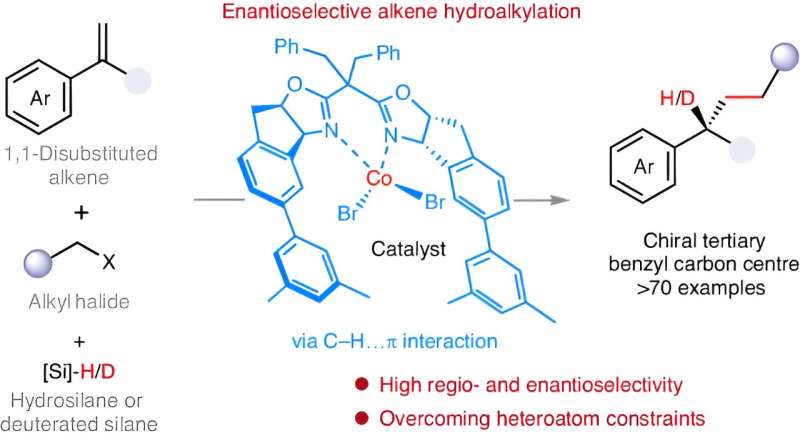
Professor Fu Yao and Associate Professor Lu Xi from the University of Science and Technology of China (USTC) have conducted a cobalt-hydride-catalyzed enantioselective hydroalkylation, enabling the efficient construction of chiral tertiary carbon centers at the benzyl position. The study has been published in Nature Synthesis.
Alkyl carbon centers serve as scaffolds for 3D configuration in organic molecules and functional materials, endowing these molecules with unique properties and functions. Alkene hydroalkylation reactions make alkyl carbon centers by reacting metal hydride species with alkenes. However, controlling the stereochemical selectivity of this reaction has been challenging.
The researchers investigated the structure and reaction regularity of alkyl metal intermediates, establishing a new model that uses C-H···π non-covalent interactions to control reaction selectivity. The π-electron system of the styrene substrate was found to form C-H···π interactions with hydrogen atoms in the catalyst, which enabled precise control over the stereochemical selectivity of the reaction.
Researchers carried out the cobalt-catalyzed hydroalkylation of alkenes with precise construction of aryl vicinal chiral alkyl carbon centers. This experiment solved the problem of uncontrollable stereochemistry in heteroatom-free alkene hydroalkylation reactions.
The hydroalkylation of alkenes with alkyl halides was applicable to a wide range of substrates and features excellent functional group compatibility. Moreover, the catalytic system enabled deuterium-labeled hydroalkylation of alkenes, which showed potential applications in the synthesis and modification of pharmaceutical molecules and natural products.
The study reveals a new synthetic pathway for chiral functional molecules such as deuterated drugs.
More information: Yan Li et al, Enantioselective alkene hydroalkylation overcoming heteroatom constraints via cobalt catalysis, Nature Synthesis (2024). DOI: 10.1038/s44160-024-00581-x
Journal information: Nature Synthesis
Provided by University of Science and Technology of China