This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Lewis-base ligand optimized electrolyte composition enhances CO₂ electrolysis performance
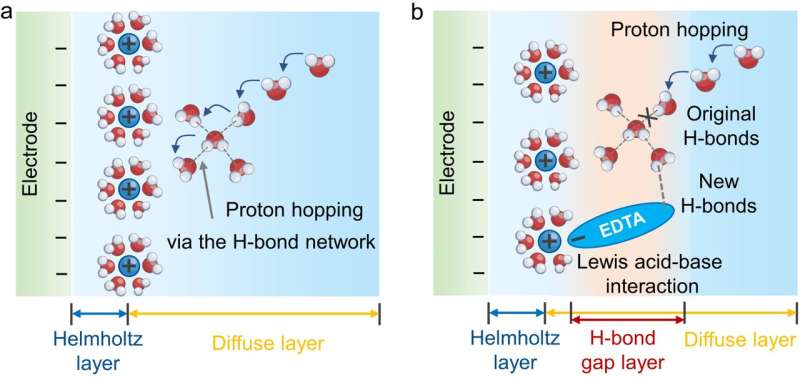
The electrode-electrolyte interface where electrocatalytic reactions occur, buried between solid-catalysts and electrolytes, involves complicated processes of electron transfer and mass diffusion under an applied electric field. Understanding the interfacial organization and possible interfacial interactions, such as those between the electrocatalysts and electrolytes or among electrolyte components, is essential for improving electrochemical performance via the co-optimization of electrocatalysts and electrolytes.
Recently, Prof. Hongliang Jiang, Prof. Cheng Lian and Prof. Chunzhong Li from East China University of Science and Technology published a research article titled "Lewis-base ligand-reshaped interfacial hydrogen-bond network boosts CO2 electrolysis" in the journal National Science Review.
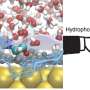
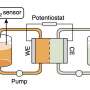
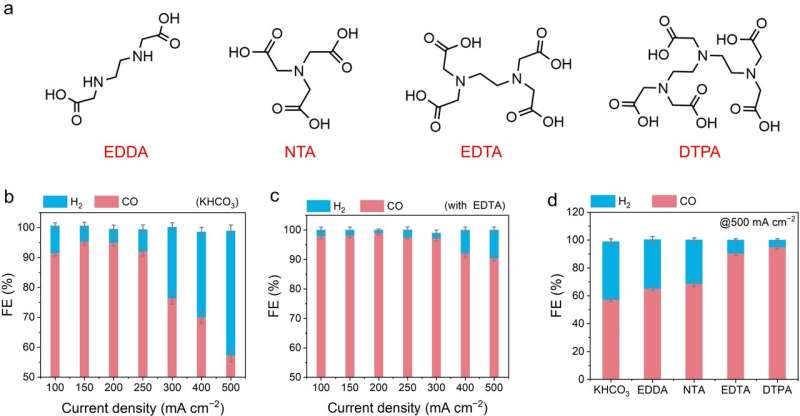
This study proposes a strategy to regulate the electrode-electrolyte interface using Lewis-base ligand molecules. It involves adding trace amounts of ethylenediaminetetraacetic acid molecules and similar ligands as electrolyte additives. In situ infrared and ab initio molecular dynamics calculations reveal the dynamic changes of ethylenediaminetetraacetic acid ligands at the electrochemical interface and their role in catalyzing CO2 reduction.
The Lewis-base ligands reconstruct the cation solvation shell through Lewis acid-base interactions and reshape the interface hydrogen-bond network by forming an H-bond gap layer. This strategy can be further extended to a series of commercial catalysts.
This study not only proposes a strategy of Lewis base ligand regulation of catalytic interfaces, but also elucidates the mechanism of Lewis base ligands in CO2 electrolysis, providing new insights into the interactions of electrolyte components in the electric double layer, and offering a new framework for understanding the organization of complex electrochemical interfaces.
More information: Wangxin Ge et al, Lewis-base ligand-reshaped interfacial hydrogen-bond network boosts CO2 electrolysis, National Science Review (2024). DOI: 10.1093/nsr/nwae218
Provided by Science China Press