This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Understanding the cation effect on the CO coupling reaction at the electrochemical interface
Recently, the National Science Review published research from Prof. Jun Cheng (Xiamen University) and Dr. Jia-Bo Le (Ningbo Institute of Materials Technology and Engineering of Chinese Academy of Sciences). The research team used ab initio molecular dynamics simulation methods to study the microscopic properties of the electrochemical interface, and then applied these findings to understand the mechanism behind the cation effect on the selectivity of C2 products in the electrocatalytic reduction of carbon monoxide.
In many electrocatalytic reactions, researchers have found that changing the electrolyte cation can significantly adjust the catalytic activity and selectivity, but there is still a debate about the microscopic mechanism involved in this process.
To address this issue, researchers used the electrocatalytic reduction of carbon monoxide as an example, systematically studying the effect of different cations (including various alkali metal ions and alkyl-ammonium ions) on the microscopic structure of the Pt(111)-COad/aqueous solution interface, and its correlation with the activity of the CO coupling reaction.
The study found that:
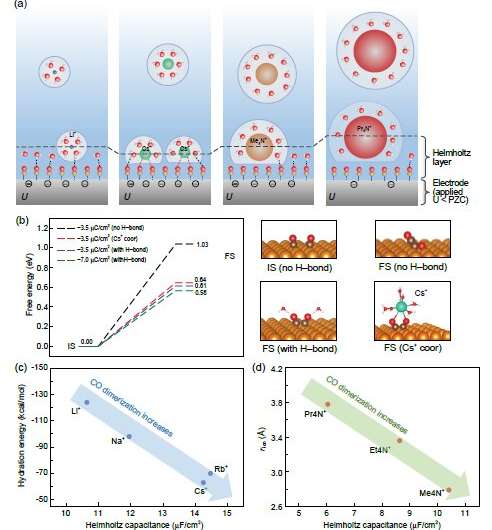
1) The solvation layer structure of the cation at the interface is related to the ion hydration energy. Ions with small hydration energy, such as Cs+, are more likely to undergo dehydration at the interface, thus coordinating with the surface-adsorbed CO molecules and affecting the adsorption stability of CO and its coupling intermediate.
2) The properties of the cation affect the double layer capacitance at the interface. Generally, ions with smaller hydration radii have larger interface capacitance, which can explain why the capacitance of alkylammonium ions is usually smaller than that of alkali metal ions. In addition, two special phenomena were discovered by researchers in this work: one is the over-screening effect of low hydration energy ions (such as Cs+), which increases the capacitance; the other is that super-sized ions (such as propyl-ammonium ions) reduce the water content of the interface, thus decreasing its dielectric screening ability and reducing the capacitance.
3) Surface reaction calculations found that the energy of the CO coupling reaction on the electrode surface is significantly correlated with surface charge, ion coordination, and hydrogen bonding with water molecules. When combined with the effect of cations on the microscopic properties of the interface, the experimental trend of different cations on the selectivity of CO electro-reduction C2 products can be well understood, namely Cs+~Rb+ > K+ > Li+ and Me4N+ > Et4N+ > Pr4N+. This work provides important guidance for the design of electro-reduction catalytic systems for carbon monoxide/carbon dioxide in the future.
More information: Jia-Bo Le et al, Molecular understanding of cation effects on double layer and its significance to CO-CO dimerization, National Science Review (2023). DOI: 10.1093/nsr/nwad105
Provided by Science China Press