This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Stud offers insights for the future design of highly efficient multi-element electrocatalysts
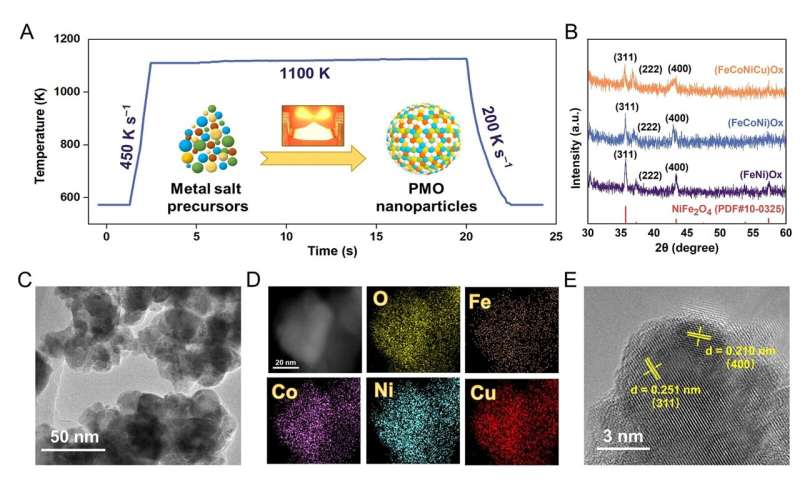
Professor Zhe Weng and Chunpeng Yang from Tianjin University published a paper titled "Unveiling multi-element synergy in polymetallic oxides for efficient nitrate reduction to ammonia" in the journal Science China Materials.
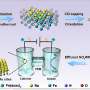
In this study, the (FeCoNiCu)Ox/CeO2 electrocatalyst for the reduction of NO3− to NH3 was prepared using the rapid Joule-heating method within a short duration. Electrochemical measurements revealed that the (FeCoNiCu)Ox/CeO2 electrocatalyst exhibited a high Faradaic efficiency for NH3 exceeding 90% in the potential range of 0 to −0.4 V vs.
RHE, along with a high NH3 yield rate of 30.3 mg h−1 cm−2. Moreover, the (FeCoNiCu)Ox/CeO2 electrocatalyst demonstrated excellent long-term stability for more than 10 h at 200 mA cm−2.
Through a series of comprehensive experiments, the individual contributions of each element and their synergistic effect have been clearly elucidated.
-
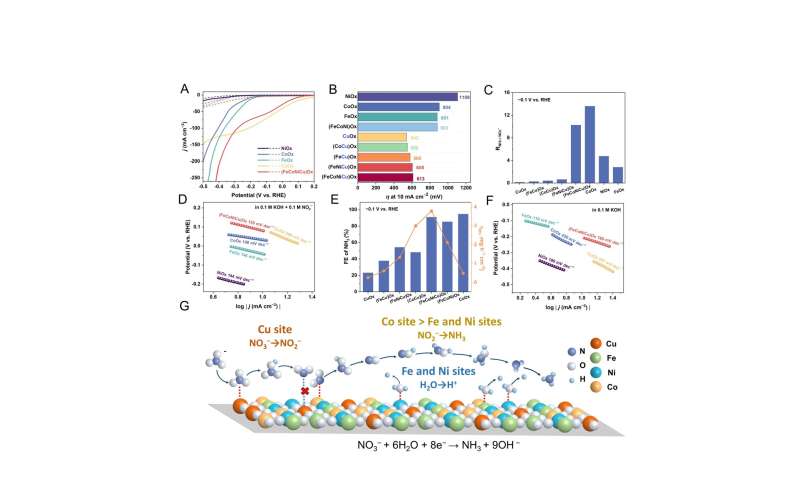
Several single/binary/ternary MOx electrocatalysts (M = Fe, Co, Ni, Cu, FeCo, FeNi, FeCu, CoCu, FeCoNi and FeNiCu) as control samples were also prepared using the same Joule-heating synthesis method and electrochemical tested. Credit: Science China Press -
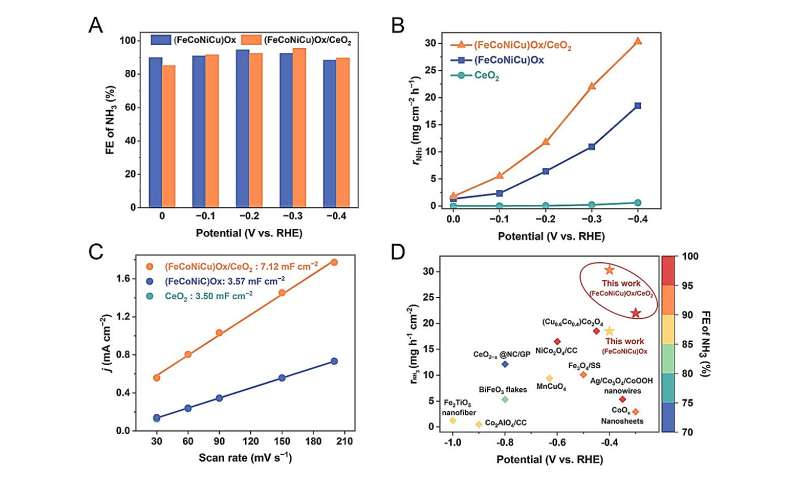
The NO3−RR performance of the (FeCoNiCu)Ox electrocatalyst was studied under different reduction potentials. The (FeCoNiCu)Ox electrocatalyst demonstrates a high FENH3 of over 90% in the wide potential range of 0 to −0.4 V vs. RHE. However, the rNH3 of the (FeCoNiCu)Ox is not as competitive compared to other oxide electrocatalysts reported in previous literatures. TEM and SEM images reveal particle agglomeration, which could affect the dispersion of active components. Previous studies have reported that incorporating CeO2 as a support in catalysts improves the uniform dispersion of active components and significantly increases the active surface area of the catalysts. Moreover, Ce has a much larger atomic radius than the other elements (Fe, Co, Ni and Cu) and thus Ce cannot incorporate the crystal structure of (FeCoNiCu)Ox even by the rapid Joule-heating method. Accordingly, a (FeCoNiCu)Ox/CeO2 composite catalyst was further successfully synthesized. Credit: Science China Press -
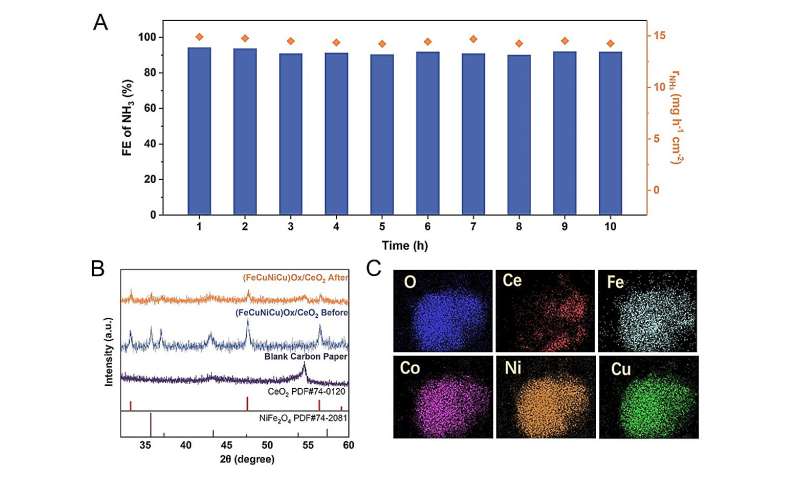
During the 10 h of electrocatalysis at 200 mA cm−2, the FENH3 and rNH3 demonstrate remarkable stability. The XRD pattern reveals that the position and shape of the peaks remain largely unaltered before and after electrocatalysis. The EDS elemental mapping of the (FeCoNiCu)Ox/CeO2 electrocatalyst after electrocatalysis clearly displays identical element distribution as before electrocatalysis. These results suggests that excellent stability of the (FeCoNiCu)Ox/CeO2 electrocatalyst. Credit: Science China Press
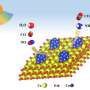
Specifically, the Cu active sites efficiently reduce NO3− to nitrite (NO2−) at low overpotential, while the adjacent Co sites facilitate the deep reduction of intermediate NO2−.
The Fe and Ni sites play a crucial role in promoting water dissociation to ensure sufficient proton supply. Simultaneously, the CeO2 component increases the active surface area of the (FeCoNiCu)Ox electrocatalyst and improves the NH3 yield rate, making it suitable for industrial applications. This work offers significant insights into the design of highly efficient multi-element electrocatalysts.
More information: Yaning Qie et al, Unveiling multi-element synergy in polymetallic oxides for efficient nitrate reduction to ammonia, Science China Materials (2024). DOI: 10.1007/s40843-024-3017-4
Provided by Science China Press