This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Scientists overcome NH3 synthesis shortcomings
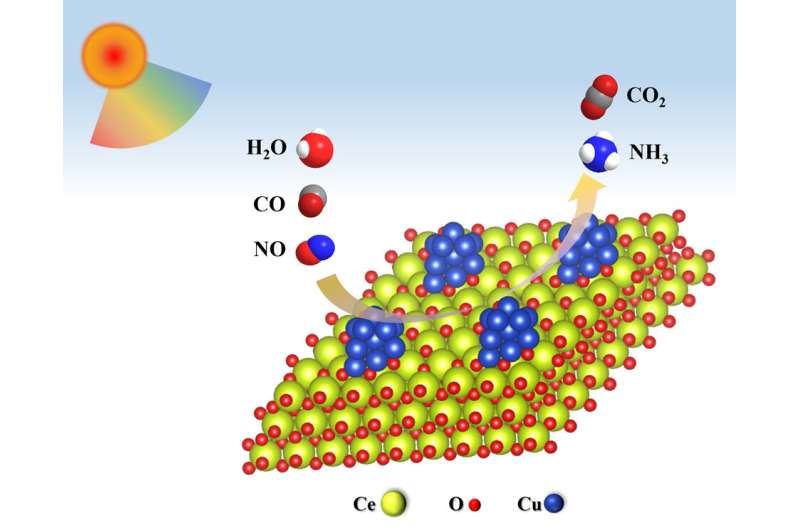
NH3 is not only the key chemical raw material for the industry but also a carbon-free fuel and mobile carrier of renewable energy in the future. So far, industrial NH3 synthesis is still dominated by the traditional Haber-Bosch reaction, which requires high temperatures of 300–500 ºC and pressures of 200–300 atm.
To overcome these shortcomings, Nanba et al. have designed the NO-CO-H2O reaction system. In this reaction, NO was used as the raw material and reduced to NH3 by H2O and toxic gases CO. The reaction equation is as follows: NO + 2.5CO + 1.5H2O → NH3 + 2.5CO2 (ΔH298.15K= -414.86 kJ·mol-1)
Recently, their research found that the NO-CO-H2O reaction can be approximately decomposed into a series reaction of WGSR of CO-H2O and reduction of NO-H2. The results were published in the Chinese Journal of Catalysis.
When the incident photon frequency of incident light matches the vibration frequency of noble metal nanoparticles, the nanoparticles have strong absorption of photon energy and local surface plasmon resonance (LSPR) occurs. The metal with the LSPR effect can excite high-energy hot electrons and holes, which helps to activate the reactants, thus reducing the reaction energy and increasing the reaction rate. As a rare non-noble metal with an LSPR effect, Cu has been widely used in CO hydrogenation reactions.
Cu/CeO2 was prepared by a simple wet impregnation method and the reactivity of NO reduction to NH3 by CO in a photothermal synergistic system was studied. As expected, high activity was obtained over Cu/CeO2 under visible light irradiation. The LSPR effect of Cu nanoparticles can increase the NH3 yield under mild conditions.
Recently, a research team led by Prof. Wenxin Dai from Fuzhou University, China, reported a photothermal catalytic system comprising Cu/CeO2 that was applied to the reaction between NO, CO and H2O for the production of NH3 under visible-light irradiation. High NO conversion (94.4%) and NH3 selectivity (66.5%) were achieved over Cu/CeO2 in the presence of H2O at 210°C. Visible light further improved the conversion of NO (97.7%) and selectivity for NH3 (69.1%).
The quasi-situ EPR and in-situ DRIFTS results indicated that CO initially reacts with H2O to form an HCO3* intermediate, which then decomposes into CO2 and activated H*. Finally, NO reacts with activated H* to produce NH3. The localized surface plasmon resonance effect of Cu nanoparticles induced by visible light promotes the decomposition of HCO3* to CO2 and H*, while regenerating oxygen vacancies (OVs, H2O activation sites) at the CeO2 sites, resulting in enhanced NH3 production.
More information: Xinjie Song et al, NH3 synthesis via visible-light-assisted thermocatalytic NO reduction by CO in the presence of H2O over Cu/CeO2, Chinese Journal of Catalysis (2023). DOI: 10.1016/S1872-2067(23)64439-0
Provided by Chinese Academy of Sciences