This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Tandem single-atom electrocatalyst realizes reduction of CO2 to ethanol
The electrochemical CO2 reduction reaction (CO2RR) into carbon-based fuels provides a promising strategy to mitigate CO2 emission and promotes the utilization of renewable energy.
The Cn (n≥2) liquid products are desirable because of their high energy densities and ease of storage. However, manipulation of C–C coupling pathway remains a challenge due to the limited mechanistic understanding.
Recently, a research group led by Profs. Zhang Tao and Huang Yanqiang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has developed a Sn-based tandem electrocatalyst (SnS2@Sn1-O3G), which could reproducibly yield ethanol with a Faradaic efficiency of up to 82.5% at -0.9 VRHE and a geometric current density of 17.8 mA/cm2.
The study was published in Nature Energy on Oct. 30.
The researchers fabricated the SnS2@Sn1-O3G through solvothermal reaction of SnBr2 and thiourea on a three-dimensional carbon foam. The electrocatalyst comprised SnS2 nanosheets and atomically dispersed Sn atoms (Sn1-O3G).
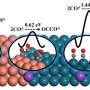
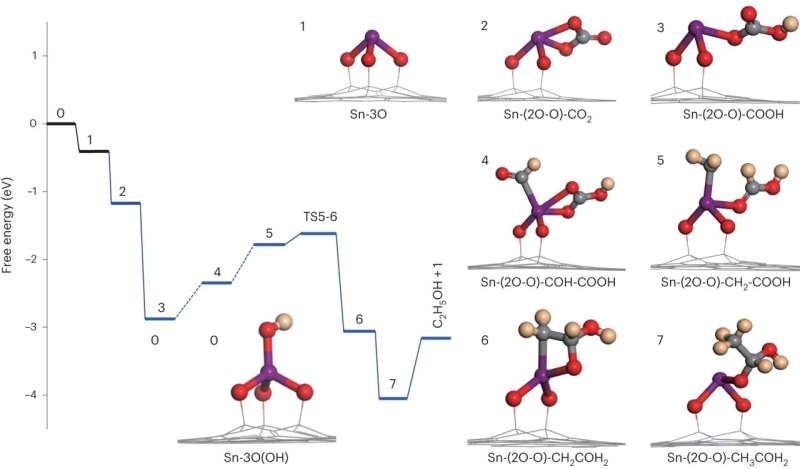
Mechanistic study showed that this Sn1-O3G could respectively adsorb *CHO and *CO(OH) intermediates, therefore promoting C–C bond formation through an unprecedented formyl-bicarbonate coupling pathway.
Moreover, by using isotopically labeled reactants, the researchers traced the pathway of C atoms in the final C2 product formed over the catalyst of Sn1-O3G. This analysis suggested that the methyl C in the product comes from formic acid whereas the methylene C was from CO2.
"Our study provides an alternative platform for C–C bond formation for ethanol synthesis and offers a strategy for manipulating CO2 reduction pathways towards desired products," said Prof. Huang.
More information: Jie Ding et al, A tin-based tandem electrocatalyst for CO2 reduction to ethanol with 80% selectivity, Nature Energy (2023). DOI: 10.1038/s41560-023-01389-3
Journal information: Nature Energy
Provided by Chinese Academy of Sciences