This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New strategy boosts selective carbon monoxide electrolysis to acetate
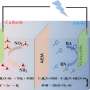
Alkaline CO2 electrolysis can produce multicarbon (C2+) products such as ethylene and acetate, yet suffers from low CO2 utilization efficiency.
Tandem electrolysis, which connects solid oxide or acidic CO2 electrolysis to CO and alkaline CO electrolysis to C2+ products in sequential electrolyzers, is a carbon-efficient route. However, to date, CO electrolysis generally shows high current density and selectivity for C2+ products, but selective generation of a specific C2+ product is still challenging.
Recently, a research team led by Profs. Wang Guoxiong and Gao Dunfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has proposed a new strategy by constructing metal-organic interfaces for CO electrolysis to acetate with high selectivity.
This study was published in Angewandte Chemie International Edition on Sept. 25.
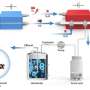
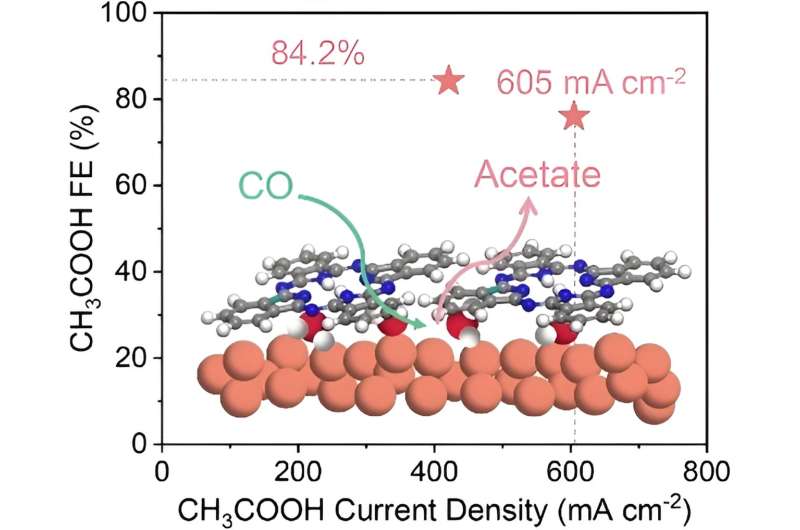
The researchers tuned the reaction microenvironments surrounding catalytically active sites by constructing Cu-organic interfaces through in-situ electrochemical reconstruction of molecular Cu complexes. Benefiting from the favorable reaction microenvironment, they achieved good catalytic performance for CO electrolysis to acetate, in terms of current density, Faradaic efficiency, and yield.
With a copper phthalocyanine (CuPc) electrode measured in a home-made alkaline membrane electrode assembly (MEA) electrolyzer, they obtained an acetate Faradaic efficiency as high as 84.2% and an acetate carbon selectivity of 92.1% at 500 mA cm-2. The maximum acetate partial current density and formation rate reached 605 mA cm-2 and 0.38 mmol min-1, respectively, translating into an acetate yield as high as 63.4%.
The Cu-organic interface created a favorable reaction microenvironment that enhanced *CO adsorption, lowered the energy barrier for C-C coupling, and facilitated the formation of CH3COOH over other multicarbon products, thus rationalizing the selective acetate production.
"Our study highlights the potential of constructing metal-organic interfaces for tailoring reaction microenvironments for highly selective production of a specific C2+ product from CO electrolysis," said Prof. Gao.
More information: Youwen Rong et al, Directing the Selectivity of CO Electrolysis to Acetate by Constructing Metal‐Organic Interfaces, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202309893
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences