This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Deciphering the journey from stem cells to neurons: Study reveals continuous gene expression changes behind the switch
RIKEN researchers have uncovered how gradual changes in gene-expression patterns drive a cell-fate switch in neural stem cells (NSCs) in mice.
All the major neuronal types of cells in the central nervous system are created through the division of NSCs into intermediate progenitor cells (IPCs), which in turn divide into mature neurons.
The transition from NSCs to IPCs is considered to be a discrete event since IPCs lack the same multipotency and radial shape of NSCs and no cells exhibit intermediate features between NSCs and IPCs. However, RNA-sequencing analyses of single cells has shown that gene expression changes continuously from NSCs to IPCs.
Thus, while the conversion appears sudden, the underlying changes in gene expression proceed gradually during the differentiation process.
Ryoichiro Kageyama, director of the RIKEN Center for Brain Science, was intrigued by this apparent conflicting evidence. "We wanted to reconcile these contradictory observations," he says.
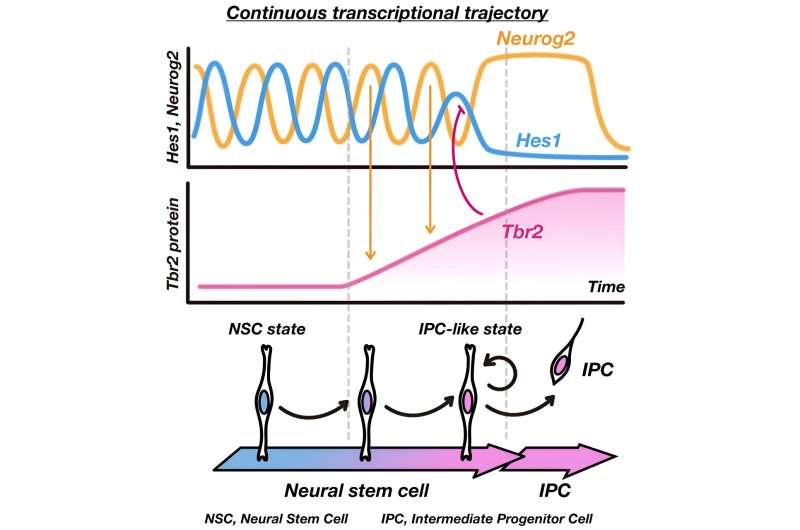
Transcription factors are proteins that control the expression of genes. Previously, Kageyama's team had shown that the transcription factors that regulate NSC differentiation are expressed in an oscillatory manner. In particular, oscillations in the expression of the transcription factor Hes1, which represses the expression of genes that promote neuronal cell fate such as Neurog2, keep NSCs alternating between an NSC state and an IPC-like state.
"NSCs with the IPC-like state differentiate into IPCs after cell division," notes Kageyama.
Now, Kageyama and two colleagues have unmasked the mechanism by which Hes1 expression is suppressed to induce an IPC-like state in mouse NSCs during development of the cerebral cortex.
The trio found that Hes1-regulated Neurog2 oscillations result in the accumulation of T-box brain protein 2 (Tbr2), a transcription factor that binds to the Hes1 promoter to switch off Hes1 expression. The study is published in the journal Developmental Cell.
"When Tbr2 reaches a certain level, it effectively suppresses Hes1, which drives IPC differentiation," says Kageyama.
Temporarily eliminating Tbr2 expression caused an increase in Hes1 expression and reduced NSC differentiation, confirming that Tbr2 plays a key role in driving the gradual conversion of NSCs into IPCs.
The discovery that NSCs exhibit differentiated-cell-like gene-expression patterns as well as those typical in stem cells was surprising. "The gene-expression patterns of NSCs are more diverse than previously thought," says Kageyama.
Since current data indicates that oscillating gene-expression states are important for multipotency and efficient proliferation of NSCs, Kageyama's team intends to explore how these oscillating gene-expression states promote cell-cycle progression in NSCs.
More information: Hiromi Shimojo et al, The Neurog2-Tbr2 axis forms a continuous transition to the neurogenic gene expression state in neural stem cells, Developmental Cell (2024). DOI: 10.1016/j.devcel.2024.04.019
Journal information: Developmental Cell
Provided by RIKEN