This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How can surface morphology change selectivity in electrocatalysis?
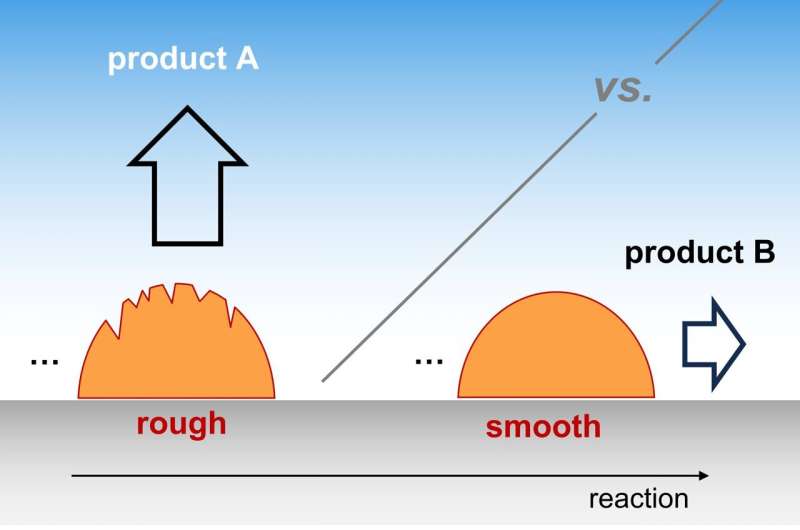
The Theory Department of the Fritz Haber Institute calls attention to catalyst morphology as a key factor in determining what product is being formed during an electrocatalytic reaction. The analysis, published in Nature Catalysis, explores how the "roughness" of a catalyst surface changes the selectivity for a number of technologically important reactions, including the electrochemical conversion of CO2 into fuels and H2O formation in fuel-cells.
The results offer a new perspective on how catalyst design could optimize electrochemical processes, while challenging the traditional picture that focuses entirely on the nature of the active site at the atomic level.
Catalysis plays a pivotal role in the chemical industry, significantly influencing numerous facets of everyday life such as plastic generation, drug development, and the manufacture of fertilizers. Heterogeneous electrocatalysis, in particular, is at the heart of developing sustainable energy technologies as it enables the carbon-free production of fuels and chemicals through renewable electricity. Here, chemical transformations require only mild conditions of temperature and pressure as they are driven by charge transfer at the solid-liquid interface.
One of the key objectives of the team's research is to elucidate catalyst selectivity, the origin of which often remains poorly understood especially in the field of electrocatalysis.
This analysis focuses on a microscopic mechanism where a certain reaction intermediate escapes the catalyst surface to be detected as an early, partially-converted product. A new, multi-scale kinetic model shows how selectivity depends upon the rate of species' transport through the electrolyte and quantifies the influence from the density of catalytically active sites, also known as catalyst roughness. Despite its simplicity, the model is able to reproduce a series of trends found in the experimental literature.
This result demonstrates the generality of the proposed mechanism and establishes roughness as a key descriptor of catalyst morphology across all relevant length scales. The insight improves fundamental understanding of reaction mechanisms in electrocatalysis, while suggesting new paths ahead for optimizing catalyst selectivity and long-term operation.
More information: Hendrik H. Heenen et al, Exploring mesoscopic mass transport effects on electrocatalytic selectivity, Nature Catalysis (2024). DOI: 10.1038/s41929-024-01177-6
Journal information: Nature Catalysis
Provided by Max Planck Society