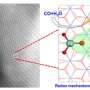
Dual‐site collaboration boosts electrochemical nitrogen reduction on Ru‐S‐C single‐atom catalyst
Ammonia (NH3) is an important fertilizer and chemical for human society; however, its production by the traditional Haber-Bosch process consumes substantial fossil fuel energy and produces massive carbon dioxide emissions. Powered by renewable energy, electrocatalytic reduction of nitrogen (N2) to NH3 under eco-friendly and mild conditions provides a highly attractive solution for carbon neutrality.
Despite recent significant progress, electrocatalytic nitrogen reduction reaction (eNRR) still suffers from limited selectivity and activity. This is due to the super-stability of N≡N triple bond. Theoretical and experimental efforts have demonstrated that the electrocatalysts always face a significant challenge to effectively activate N2 and accomplish the first protonation of N2 to form NNH* in the rate-determining step (RDS).
One strategy to break the above limitation of eNRR is to involve multi-reaction sites in catalytic reactions, just like the catalytically active sites in talented metalloenzymes. For instance, in Fe nitrogenase, the S atom adjacent to the Fe center functions as a co-catalytic site to bind protons (H*), which electrostatically activates the N2 molecule adsorbed by the Fe center to the optimum state and provides H* for the hydrogenation of N2.
Such a close collaboration between the metal center and its coordination atoms enables the nitrogenase to achieve ultrahigh activity and selectivity. Therefore, one can expect that the synergetic work of multiple catalytic sites on the catalyst surface can significantly enhance the activity and selectivity of eNRR.
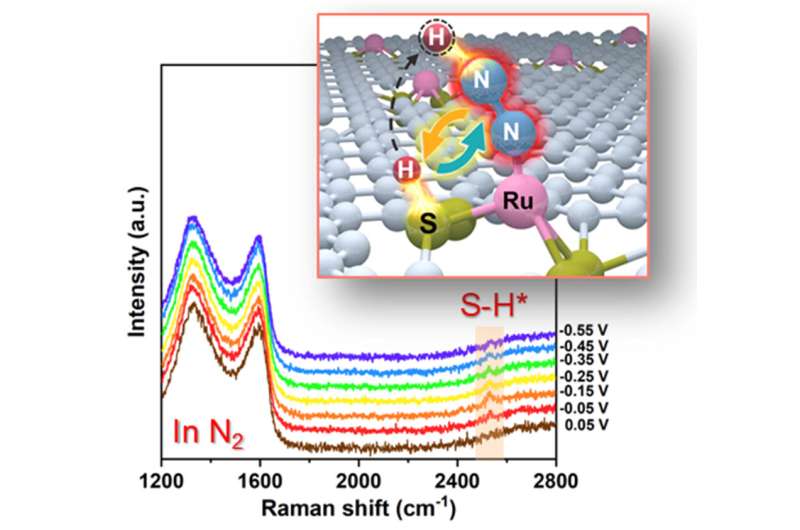
Recently, a research team led by Prof. Tao Ling from Tianjin University, China, proposed to realize a synergetic work of multi-reaction sites to overcome the limitation of sustainable NH3 production. Herein, using ruthenium-sulfur-carbon (Ru-S-C) catalyst as a prototype, the researchers showed that the Ru/S dual-site cooperated to catalyze eNRR at ambient conditions.
With the combination of theoretical calculations, in situ Raman spectroscopy, and experimental observation, the researchers demonstrated that such Ru/S dual-site cooperation greatly facilitated the activation and first protonation of N2 in the rate-determining step of eNRR. As a result, Ru-S-C catalyst exhibited significantly enhanced eNRR performance compared with the routine Ru-N-C catalyst via a single-site catalytic mechanism.
The specifically designed dual-site collaborative catalytic mechanism could offer new opportunities for advancing sustainable NH3 production.
The results were published in Chinese Journal of Catalysis..
More information: Liujing Yang et al, Dual-site collaboration boosts electrochemical nitrogen reduction on Ru-S-C single-atom catalyst, Chinese Journal of Catalysis (2022). DOI: 10.1016/S1872-2067(22)64136-6
Provided by Chinese Academy of Sciences