This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New strategy proposed for direct synthesis of alcohols from reductive hydroformylation of alkenes
Linear alcohols, as an important class of chemical products, are indispensable in the production of food, pharmaceuticals, plasticizers, and lubricants. Direct conversion of readily available alkenes into high-value alcohols is important but challenging in both organic synthesis and industry.
One-pot reductive hydroformylation of alkenes provides a facile and atom-economic method for the synthesis of single-carbon homologous alcohols. However, the reaction catalyzed by a stable and efficient heterogeneous catalyst has been underexplored.
To address the issue, researchers from the Qingdao Institute of Bioenergy and Bioprocess Technology of the Chinese Academy of Sciences have developed a confined trinuclear Ru site in phosphine-incorporated porous organic polymers for the direct synthesis of alcohols from reductive hydroformylation of alkenes. Their study was published in ACS Catalysis on March 12.
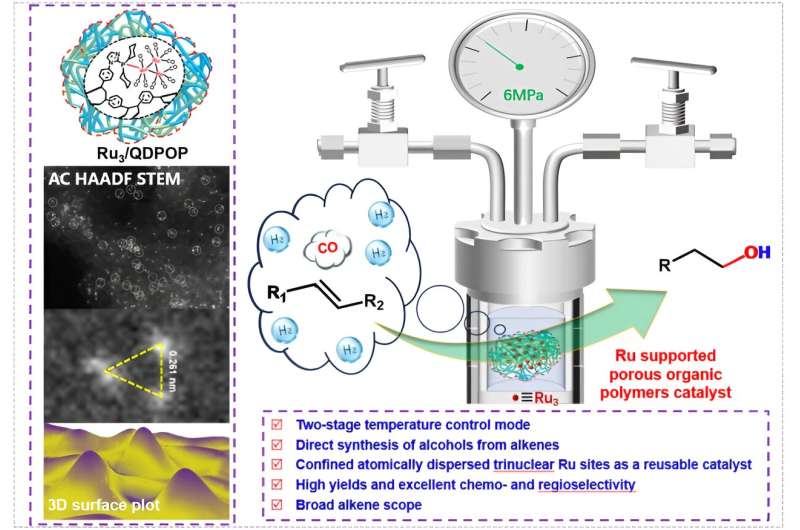
The researchers constructed a bench-stable porous organic polymer (POP) by incorporating a specific monophosphine ligand into the scaffold, which serves as both solid ligand and support to prepare a heterogeneous Ru catalyst for one-pot reductive hydroformylation.
The monophosphine ligand helps to stabilize atomically dispersed trinuclear Ru sites on POP, resulting in a heterogeneous Ru catalyst with a catalytic performance comparable to its homogeneous counterpart under the same conditions. The catalyst could be easily separated for successive reuse without a significant loss of activity and selectivity.
In addition, a two-stage temperature-programmed strategy was developed to greatly enhance the selectivity to linear alcohols, which is broadly applicable to terminal, internal, and functionalized olefins.
In this strategy, olefin was converted to the corresponding linear aldehyde at a relatively lower reaction temperature while minimizing the side reactions of isomerization and hydrogenation, thus ensuring for the production of linear alcohol in the second stage with high efficiency and excellent selectivity.
Under optimal reaction conditions, the reductive hydroformylation of 1-hexene as a model reaction was fully completed along with a selectivity to 1-hexanol of up to 95% and a linear to branched alcohol ratio of up to 30.
This work represents a significant advance in the development of heterogeneous catalysts for the direct synthesis of linear alcohols from widely available alkenes.
More information: Yinghao Zhu et al, Confined Trinuclear Ru Sites in Phosphine-Incorporated Porous Organic Polymers for the Direct Synthesis of Alcohols from Reductive Hydroformylation of Alkenes, ACS Catalysis (2024). DOI: 10.1021/acscatal.3c06221
Journal information: ACS Catalysis
Provided by Chinese Academy of Sciences