This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
In situ microscopy gives atomic-level view of mitochondria
Novel high-resolution microscopy technology is allowing researchers to see for the first time the dynamic processes of respiration in a native membrane environment at the atomic level. The new technique could help researchers better understand what's happening inside mitochondria and other organelles of diseased cells and identify new, more precise drug targets.
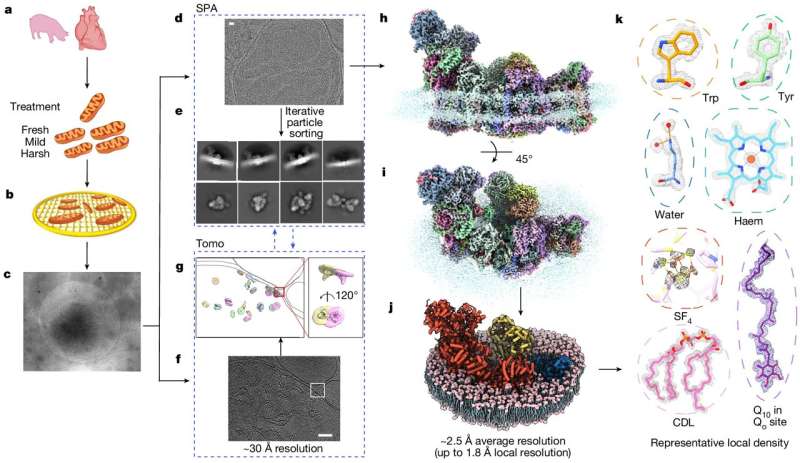
In a new study published in Nature on May 29, researchers developed a novel imaging approach by integrating two microscopy techniques—single-particle analysis and cryo-electron tomography (cryo-ET)—to image mitochondria from animal models.
They also developed original computational approaches to tackle bottlenecks to high-resolution cryo-electron microscopy (cryo-EM) analysis of images from the highly crowded environment.
The technology enabled the team to view assemblies of proteins in their native environment within the organelle with unprecedented resolution. They also examined how inducing disease in these models altered the structures of these proteins inside the mitochondrial membrane. Their work paves the way for viewing the composition of molecules within cells at the atomic level.
"Previously, our technology was not able to reveal how interactions occur," says Jack Zhang, Ph.D., assistant professor of molecular biophysics and biochemistry and principal investigator of the study. "But in this case, we see the atoms, we see the structures of molecules, and we see how everything interacts, giving us an entirely new understanding of the mechanisms in the cellular environment."
Combining two microscopy concepts to generate high-resolution images
Currently, structural biologists are limited by either the resolution or achievable scales of targets of available microscopy techniques. "We haven't even been able to distinguish protein subunits [using conventional approaches], let alone atomic details in cellular environments," says Zhang.
The Yale team's feat was made possible by combining the concepts of single-particle analysis cryo-EM and cryo-ET technologies, along with new development of image analysis methodology to analyze high-resolution cellular cryo-EM images at the cryo-ET scale.
"Single-particle analysis is great for imaging individual molecules, but not for cellular structures," Zhang explains. "Cryo-electron tomography is a perfect tool to study cellular structures, but its resolution is typically limited to several nanometers ."
They overcame this technical challenge by developing an approach to image cryo-ET targets under single-particle mode, while employing cryo-ET reconstructions as initial references for subsequent single-particle alignment and classification.
Another limitation of conventional single-particle cryo-EM is that it typically requires researchers to isolate and purify proteins of interest before freezing and mounting them onto cryo-EM grids, thus removing the molecules from their native environment.
This process disrupts weakly assembled higher-order biological complexes, as well as reactions under physiological conditions. This is particularly important in the case of membrane proteins, because purified samples using detergent would completely disrupt the native membrane environment, which is crucial to their normal functions.
By combining these microscopy approaches and developing novel computational methods, the team was able to create images both at single-particle resolution and at a cryo-ET scale, and then bridge the two using innovative algorithms, allowing researchers to view molecular structures with an unparalleled level of detail.
Notably, their technique allowed them, for the first time, to view membrane protein structures within their native mitochondria—and to view the proteins' natural assembly and function within the whole organelle.
Researchers visualize mitochondrial supercomplexes in atomic detail
Using their new microscopy technique, the researchers directly imaged mitochondria from animal heart models to examine mitochondrial respiratory supercomplexes, which are assemblies of proteins living in the organelle's inner membrane and playing a vital role in energy production.
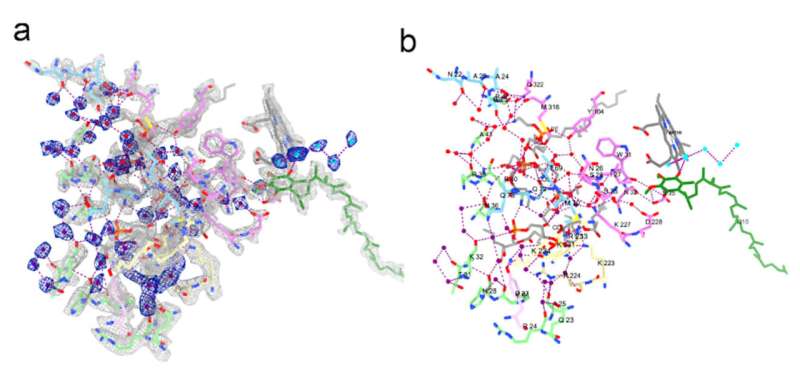
For the first time, the team was able to view the structures of these supercomplexes in atomic detail without having to isolate them from the mitochondria. It was especially striking that the resolution even allowed for direct observation of individual water molecules associated with these complexes.
Because all these supercomplexes were in their native environment, which is distinct from purified proteins, the team was able to visualize the structures of the numerous reactive intermediates while they are functioning within mitochondria at an atomic level.
"Using a novel classification strategy, we see how proteins are reacting to their environment and captured different intermediate states," says Zhang.
Conventional approaches only provide location information of molecules or their overall shapes at low resolution in cellular environments. This new approach has substantial pharmaceutical and clinical implications. "It can help us study how molecules react to certain drugs in their native cells," Zhang says.
Paving the way to novel, more effective drug development
This microscopy technology could also be groundbreaking in helping researchers understand the underlying mechanisms of various diseases. To explore this further, the Yale team created animal heart models of various states of myocardial ischemia, a cardiovascular condition in which a blockage obstructs blood flow to the heart.
They found that the different stages of disease induced significant changes of conformational distribution, and changes in shape, in the supercomplexes along with their native membranes.
Experts who reviewed the Zhang team's work on behalf of Nature wrote, "This groundbreaking work sets new standards in structural biology," and, "Future work will exploit the huge potential of this new approach."
Moving forward, the Yale team's technique can give researchers an entirely new look into what is happening inside normal and diseased cells, which could eventually pave the way for the development of highly accurate therapies that target newfound molecular pathways or structures.
This is particularly important in the case of membrane proteins, as they are crucial drug targets due to their key roles in physiological and pathological processes, including cell-cell communication, pathogen-host interactions, signal transduction, their accessibility for drugs, high specificity and selectivity, diverse target classes, and significant therapeutic potential.
Zhang's team is already looking ahead to leading this technology to study various disease models, including animal models and, potentially, donor tissues, to investigate what is happening inside the cells in these different contexts. "By expanding our studies to medical applications, we can provide unprecedented detail of what's happening in diseased cells so that we can provide better drugs and treatment."
More information: Wan Zheng et al, High-resolution in situ structures of mammalian respiratory supercomplexes, Nature (2024). DOI: 10.1038/s41586-024-07488-9
Journal information: Nature
Provided by Yale University


















