This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New method optimizes lithium extraction from seawater and groundwater
As the electric vehicle market booms, the demand for lithium—the mineral required for lithium-ion batteries—has also soared. Global lithium production has more than tripled in the last decade. But current methods of extracting lithium from rock ores or brines are slow and come with high energy demands and environmental costs. They also require sources of lithium which are incredibly concentrated to begin with and are only found in a few countries.
Now, researchers at the University of Chicago Pritzker School of Molecular Engineering (PME) have optimized a new method for extracting lithium from more dilute—and widespread—sources of the mineral, including seawater, groundwater, and "flowback water" left behind from fracking and offshore oil drilling.
"Right now there is a gap between the demand for lithium and the production," said Chong Liu, Neubauer Family Assistant Professor of Molecular Engineering and senior author of the new work, published in Nature Communications. "Our method allows the efficient extraction of the mineral from very dilute liquids, which can greatly broaden the potential sources of lithium."
In the new research, Liu and her colleagues showed how certain particles of iron phosphate can most efficiently pull lithium out of dilute liquids. Their new findings could hasten an era of faster, greener lithium extraction.
Lithium at a cost
Today, most lithium used in lithium batteries comes from two basic extraction processes. Lithium rock ores can be mined, smashed up with heavy machinery, and then treated with acid to isolate the lithium. Lithium brine pools, on the other hand, use massive amounts of water pumped to the earth's surface and then evaporated away—over the course of more than a year—to yield dried lithium.
"These methods aren't particularly environmentally friendly to begin with, and if you start trying to work with less concentrated sources of lithium, they're going to become even less efficient," said Liu. "If you have a brine that is 10 times more dilute, you need 10 times more briny water to get the same amount of lithium."
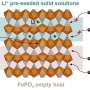
In recent years, Liu's team has spearheaded a completely different method to get lithium out of dilute liquids. Their approach isolates lithium based on its electrochemical properties, using crystal lattices of olivine iron phosphate. Because of its size, charge and reactivity, lithium is drawn into the spaces in the olivine iron phosphate columns—like water being soaked into the holes in a sponge. But, if the column is designed perfectly, sodium ions, also present in briny liquids, are left out or enter the iron phosphate at a much lower level.
In the new work, Liu and her colleagues, including first author of the new paper Gangbin Yan, a PME graduate student, tested how variation in olivine iron phosphate particles impacted their ability to selectively isolate lithium over sodium.
"When you produce iron phosphate, you can get particles that are drastically different sizes and shapes," explains Yan. "In order to figure out the best synthesis method, we need to know which of those particles are most efficient at selecting lithium over sodium."
Not too big, not too small
The research team synthesized olivine iron phosphate particles using different methods, resulting in a range of particle sizes spanning 20 to 6,000 nanometers. Then, they divided those particles into groups based on their size and used them to build electrodes that could extract lithium from a weak solution.
When iron phosphate particles were too large or too small, they discovered, they tended to let more sodium into their structures. That led to less pure extractions of lithium.
"It turned out that there was this sweet spot in the middle where both the kinetics and the thermodynamics favor lithium over sodium," said Liu.
The findings are vital to moving electrochemical lithium extraction toward commercial use. They suggest that researchers should focus on not just producing olivine iron phosphate, but producing olivine iron phosphate at the ideal particle size.
"We have to keep this desired particle size in mind as we pick synthesis methods to scale up," Liu said. "But if we can do this, we think we can develop a method that reduces the environmental impact of lithium production and secures the lithium supply in this country."
Other authors on the paper are Emory Apodaca, Suin Choi, Peter J. Eng, Joanne E. Stubbs, Yu Han, Siqi Zou, Mrinal K. Bera and Ronghui Wu of University of Chicago; Jialiang Wei and Wei Chen of Illinois Institute of Technology; and Evguenia Karapetrova and Hua Zhou of Argonne National Laboratory.
More information: Gangbin Yan et al, Identifying critical features of iron phosphate particle for lithium preference, Nature Communications (2024). DOI: 10.1038/s41467-024-49191-3
Journal information: Nature Communications
Provided by University of Chicago