This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
A high-content triethyl phosphate-based, non-flammable, and high-conductivity electrolyte for lithium-ion batteries
Safety issues related to flammable electrolytes in lithium-ion batteries (LIBs) remain a major challenge for their extended application. The use of non-flammable phosphate−based electrolytes has proven its validity in inhibiting the combustion of LIBs. However, the strong interaction between Li+ and phosphate leads to a dominant solid electrolyte interphase (SEI) with limited electronic shielding, resulting in poor Li+ intercalation at the graphite (Gr) anode when using high−phosphate−content electrolytes.
A study to address this issue was conducted by Prof. Jia Xie and Ph.D. Ziqi Zeng from the School of Electrical and Electronic Engineering, Huazhong University of Science and Technology.
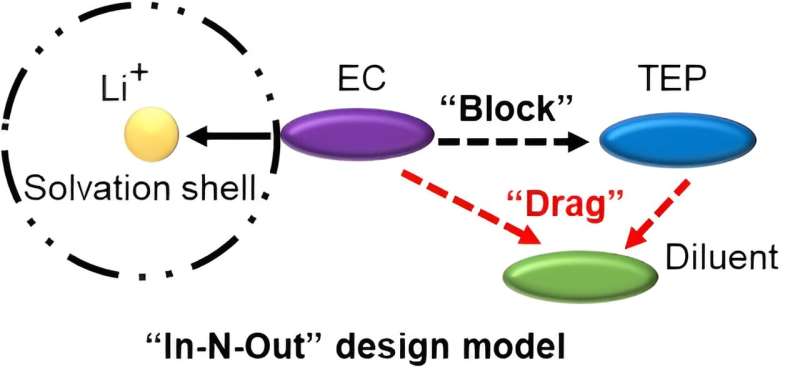
"To mitigate this issue and improve Li+ insertion, we propose an 'In−N−Out' strategy to render phosphate 'non−coordinative'. By employing a combination of strongly polar solvents for a 'block effect' and weakly polar solvents for a 'drag effect', the Li+−phosphate interaction is reduced."
"As a result, phosphate remains in the electrolyte phase ('In'), minimizing its impact on the incompatibility with the Gr electrode ('Out'). In the designed electrolyte, even if the content of TEP reaches over 60 wt.%, the Gr anode still achieves reversible Li+ de−intercalation reaction. Meanwhile, the introduction of strongly polar solvents improves the dissociation of lithium salts, thereby making the electrolyte demonstrate excellent ion conductivity (5.94 mS/cm at 30 ⁰C)," Xie says.
A few implications thus emerge for designing non−flammable electrolytes: 1) the solvents' coordination ability with Li+ could be adjusted by "In−N−Out" strategy; 2) the "drag effect" is universal interaction between weakly polar solvents and TEP, which affords more possibility for designing non−flammable electrolytes.
The study is published in the journal Science China Chemistry.
More information: Mengchuang Liu et al, "In-N-out" design enabling high-content triethyl phosphate-based non-flammable and high-conductivity electrolytes for lithium-ion batteries, Science China Chemistry (2023). DOI: 10.1007/s11426-023-1803-x
Provided by Science China Press