This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Fuel cells: Oxidation processes of phosphorous acid revealed by tender X-rays
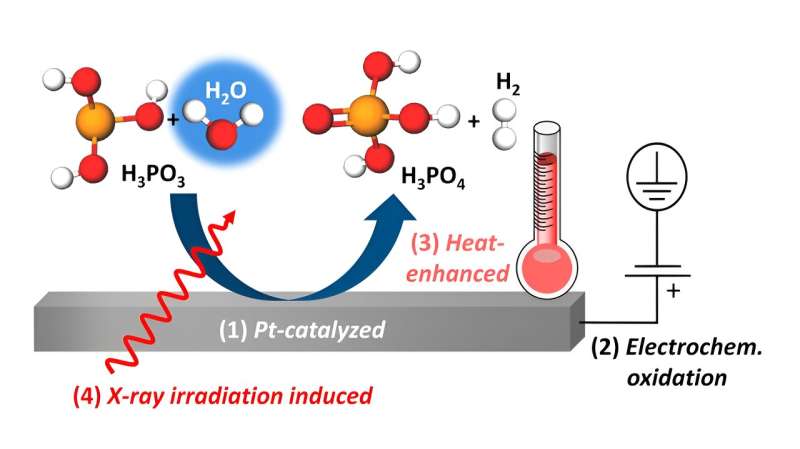
The interactions between phosphorous acid and the platinum catalyst in high-temperature PEM fuel cells are more complex than previously assumed. Experiments at BESSY II with tender X-rays have decoded the multiple oxidation processes at the platinum-electrolyte interface. The results indicate that variations in humidity can influence some of these processes in order to increase the lifetime and efficiency of fuel cells.
The work is published in the Journal of the American Chemical Society.
Hydrogen fuel cells convert chemical energy from hydrogen into electrical energy through separate reactions of hydrogen fuels and oxidizing agents (oxygen). Among hydrogen fuel cells, high-temperature polymer electrolyte membrane fuel cells (HT-PEMFCs) are attractive for micro-stationary electricity sources. One disadvantage of these HT-PEMFCs is that the phosphoric acid (H3PO4) proton conductor leaches out of the H3PO4-doped polybenzimidazole membrane and poisons the platinum catalyst.
Recent studies show further complications during the operation of the HT-PEMFC, where some of H3PO4 might be reduced to H3PO3, which may further poison the platinum catalysts, leading to a significant loss of performance.
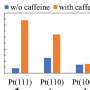
An earlier study by Prof. Dr. Marcus Bär's team showed that opposite processes also take place at the interface between Pt and aqueous H3PO3 and that the interactions between the platinum catalyst and the H3PO3/H3PO4 are very complex: While H3PO3 can lead to poisoning of the platinum catalyst, at the same time platinum might catalyze the oxidation of H3PO3 back to H3PO4.
Experiments under realistic conditions
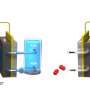
In order to investigate the oxidation behavior of aqueous H3PO3 under conditions close to HT-PEMFCs working conditions, Bär's team has now analyzed the chemical processes using an in-housed designed heatable electrochemical cell compatible for in situ tender X-ray studies at the OÆSE end-station recently set up in the Energy Materials In-situ Laboratory Berlin (EMIL).
They used intense X-ray light in the tender X-ray energy range (2 keV–5 keV), provided by the EMIL beamline at the X-ray source BESSY II. In this energy range, X-ray absorption near-edge structure spectroscopy (XANES) at the P K-edge is used to monitor oxidation processes from H3PO3 to H3PO4.
Different oxidation reactions examined
"We have thus uncovered different processes for this oxidation reaction, including platinum-catalyzed chemical oxidation, electrochemical oxidation under positive potential bias at the platinum electrode, and heat-promoted oxidation. These in situ spectroscopic results are also confirmed by ion-exchange chromatography and in situ electrochemical characterizations," explains Enggar Wibowo, first author of the study and a Ph.D. candidate in Bär's team.
"Remarkably, all of these oxidation pathways involve reactions with water, which shows that the humidity inside the fuel cell has a significant influence on these processes."
In addition, the results also point to possible improvements of the operating conditions of HT-PEM fuel cells, e.g. by controlling the humidification to oxidize the H3PO3 back to H3PO4.
"Corresponding adjustments to the operation conditions of HT-PEMFCs could be implemented to prevent catalyst poisoning by H3PO3 and enhance efficiency of those fuel cells," Wibowo points out.
"The work clarifies a key degradation pathway of fuel cells and is a contribution on the way to an H2-based energy supply," says Prof. Dr.-Ing. Marcus Bär. "It also shows the great benefit of tender X-rays, and we are looking forward to BESSY III, which aims to close the tender X-ray-gap."
More information: Romualdus Enggar Wibowo et al, Elucidating the Complex Oxidation Behavior of Aqueous H3PO3 on Pt Electrodes via In Situ Tender X-ray Absorption Near-Edge Structure Spectroscopy at the P K-Edge, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.3c12381
Journal information: Journal of the American Chemical Society
Provided by Helmholtz Association of German Research Centres