This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New technique incorporates carbon-14 in a single step for safer, more efficient drug discovery
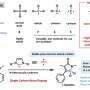
![Metal-catalyzed approaches to carbon isotope exchange with carboxylic acids. a, Prior approaches were carbonylative or carboxylative exchange methods involving the use of radioactive [14C]CO2 or [14C]CO gases (NPhth, phthalamide). b, The concept explored in this work involves exploiting a catalytic, dynamic functional group exchange to access labeled pharmaceuticals. The catalyst must affect the reversible reductive elimination of acyl chlorides without extraneous CO (step A) and undergo reversible CO de-insertion (step B) without β-hydride elimination (step C) or gaseous CO loss. c, This work has led to a broadly applicable, dynamic functional group exchange reaction for the radiolabelling of carboxylic acids with a single, simple 14C source. [Cl], in situ chlorination. Credit: Nature Chemistry (2024). DOI: 10.1038/s41557-024-01447-7 Safer, more efficient drug discovery](https://scx1.b-cdn.net/csz/news/800a/2024/safer-more-efficient-d.jpg)
McGill researchers have discovered a safer and more efficient technique for testing new drugs while they are in development.
"Because this approach is so much more streamlined, it could help accelerate this step in the drug development process and make it less dangerous since probing the distribution and fate of a drug in the body is required for any pharmaceutical candidate to be approved," says Bruce A. Arndtsen, a James McGill Professor who teaches in the Department of Chemistry at McGill and is the senior author on the paper describing the new process, published recently in Nature Chemistry.
"This research replaces what can be a days-long, dangerous, and costly process with a simple and safe one requiring only a few hours," adds José Zgheib, a Ph.D. candidate in the Arndtsen Group at McGill University who worked on the project.
Before a drug makes it to market it is tested to make sure that the molecules reach the appropriate areas of the body. This is typically done by adding a radioactive atom (e.g., carbon-14) to the drug so that its movement through the body can be traced. A bit like a GPS can be used to track the movements of animals.
But to do so currently involves a complicated, multi-step process whereby the carbon-14 atom is provided in the form of radioactive carbon monoxide or carbon dioxide gases, which are both very difficult and dangerous to work with. The gas is then incorporated into the synthesis of the medication being tested, thereby making one of its carbon atoms carbon-14.
McGill researchers have developed a new technique to incorporate carbon-14 into the drug candidates in a single step. By using a catalyst, they have been able to exchange a carbon already in the drug (in the form of a carboxylic acid) with a carbon-14 from a similar type of donor molecule. More generally, the group's work in the area highlights a potentially powerful emerging approach to modify pharmaceuticals via metal-catalyzed exchange reactions directly.
An associated research briefing is also published in the journal Nature Chemistry.
More information: Garrison Kinney et al, A metal-catalysed functional group metathesis approach to the carbon isotope labelling of carboxylic acids, Nature Chemistry (2024). DOI: 10.1038/s41557-024-01447-7
Carbon isotope exchange for pharmaceutical radiolabelling through metal-catalysed functional group metathesis, Nature Chemistry (2024). DOI: 10.1038/s41557-024-01449-5
Journal information: Nature Chemistry
Provided by McGill University