This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
This one-atom chemical reaction could transform drug discovery
Pharmaceutical synthesis is often quite complex; simplifications are needed to speed up the initial phase of drug development and lower the cost of generic production. Now, in a study recently published in Science, researchers from Osaka University have discovered a chemical reaction that could transform drug production because of its simplicity and utility.
Pharmaceuticals generally contain a few tens of atoms and a similar number of chemical bonds between the atoms. Thus, designing complex drug architectures from simple precursors using the techniques of organic chemistry usually requires careful planning and tedious, incremental steps.
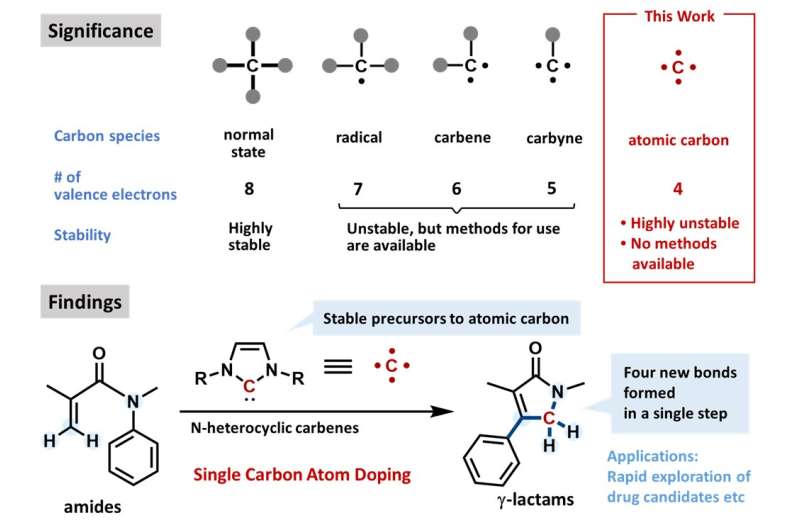
The gold standard in drug synthesis is to create, in one step, as many chemical bonds as possible. In principle, adding one carbon atom—by forming four bonds in one step—to a drug precursor can be a means of doing so. Unfortunately, atomic carbon is generally too unstable for use in common chemical reaction conditions. This is the problem that the researchers sought to address.
"Because atomic carbon is too unstable for use in organic synthesis, reagents such as dihalocarbenes are basically all that's available as atomic carbon equivalents," explains Miharu Kamitani, the lead author of the study. "We have expanded the toolkit for such reactions and have applied our technique to an established pharmaceutical."
The Osaka University researchers' discovery is based on a class of molecules known as N-heterocyclic carbenes. By a chemical process known as resonance, these molecules contain a stabilized version of a carbon atom equivalent. By a straightforward reaction with alpha, beta-unsaturated amides (an important molecule in cancer progression), various gamma-lactams (cyclic molecules that are common in antibiotics) were produced in one step, often in greater than 60% yield.
Particularly noteworthy is a one-step chemical modification of aminoglutethimide—a drug for treating seizures and other conditions—in 96% yield. Thus, even complex drugs can be modified for drug targeting and activity studies, as well as a myriad of other procedures that are otherwise synthetically complex aspects of drug discovery.
"Pharmaceutical companies are always on the lookout for straightforward reactions that achieve complex chemical transformations," says Mamoru Tobisu, senior author of the study. "We envision that our single carbon atom doping reaction will be broadly useful in this context."
This work succeeded in using an atomic carbon equivalent to form four chemical bonds in one step, synthesize pharmaceutically useful chemical architectures, and fundamentally transform the chemical nature of an established drug molecule.
The Osaka University researchers' approach will be useful for quickly preparing potential pharmaceuticals, which will speed up drug research and development as well as production of currently established drugs—especially if the approach is extended to additional classes of transformations in organic chemistry.
The article, "Single carbon atom transfer to α,β-unsaturated amides from N-heterocyclic carbenes," will be published in Science.
More information: Miharu Kamitani et al, Single carbon atom transfer to α,β-unsaturated amides from N-heterocyclic carbenes, Science (2023). DOI: 10.1126/science.ade5110. www.science.org/doi/10.1126/science.ade5110
Journal information: Science
Provided by Osaka University