This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers harness the sun to produce hydrogen gas from water
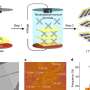
![Mechanisms of H2 evolution. a, Overview of bimetallic (homolytic) and monometallic (heterolytic) pathways for H2 evolution. b, Proposed mechanism of H2 evolution photoelectrocatalysis by the single-component catalyst [Cp*Ir(bpy)H]+. M, metal; L, ligand; e–, electron. Credit: Nature Chemistry (2024). DOI: 10.1038/s41557-024-01483-3 Researchers harness the sun to produce clean energy alternative](https://scx1.b-cdn.net/csz/news/800a/2024/researchers-harness-th.jpg)
A team of chemistry researchers at the University of North Carolina at Chapel Hill has developed a unique approach to harnessing the sun's energy to produce hydrogen gas, a potential clean energy source, from water, according to a paper published in Nature Chemistry.
Led by UNC-Chapel Hill chemist Alexander Miller, the study, "Catalyst Self-Assembly Accelerates Bimetallic Light-driven Electrocatalytic H2 Evolution in Water," investigates a system that uses light and electricity to split water into its constituent elements—hydrogen and oxygen.
"What we found is you can induce these catalysts to self-assemble into these globules, which become better at absorbing light and at making the chemical bonds to produce hydrogen," said Miller. "This research represents a significant contribution to the field of catalysis and paves the way for the development of efficient and sustainable energy technologies."
Miller, a professor of chemistry in the College of Arts and Sciences, was joined by Marc ter Horst, research professor of the nuclear magnetic resonance core laboratory; Isaac Cloward, a graduate research assistant; Tamara Jurado, a postdoctoral research associate; Tianfei Liu, a postdoctoral research associate; and former members of his lab: Annabell Bonn, Matthew Chambers, and Catherine Pitman.
The researchers discovered that molecular structures caused the catalysts—molecules that accelerate a chemical reaction without themselves being consumed in the process—to huddle together to form micelles, which are globules akin to oily deposits on the surface of water when olive oil is added to it.
Water-splitting is a key process in renewable energy technologies, particularly in the production of hydrogen as a clean and sustainable fuel. Hydrogen obtained from water can be used for fuel cells, combustion engines and other applications, with the only byproduct being water vapor.
"Water-splitting has the potential to store solar energy in the form of chemical bonds, addressing the intermittent nature of solar power generation," said Miller. "Research into efficient and cost-effective water-splitting methods is a significant area of interest in the field of renewable energy and sustainable development."
The researchers also used a special technique called dynamic light scattering, also known as photon correlation spectroscopy, to measure the size of the catalysts by analyzing the fluctuations in the intensity of scattered light. This non-invasive technique provided valuable information about the size, shape and distribution of the catalysts.
Bigger micelles produced hydrogen more quickly. They also used an analytical tool called nuclear magnetic resonance spectroscopy, which confirmed that within those particles, the catalysts were close to each other.
"We want to capture the energy in sunlight and instead of turning it into electricity, like a solar panel on your roof, we want to generate a fuel that we can store and use on demand for driving a car, charging a battery, running lights," said Miller. "That's the big picture."
More information: Isaac N. Cloward et al, Catalyst self-assembly accelerates bimetallic light-driven electrocatalytic H2 evolution in water, Nature Chemistry (2024). DOI: 10.1038/s41557-024-01483-3
Journal information: Nature Chemistry
Provided by University of North Carolina at Chapel Hill