This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Greener, cheaper method to accelerate chemical reactions developed
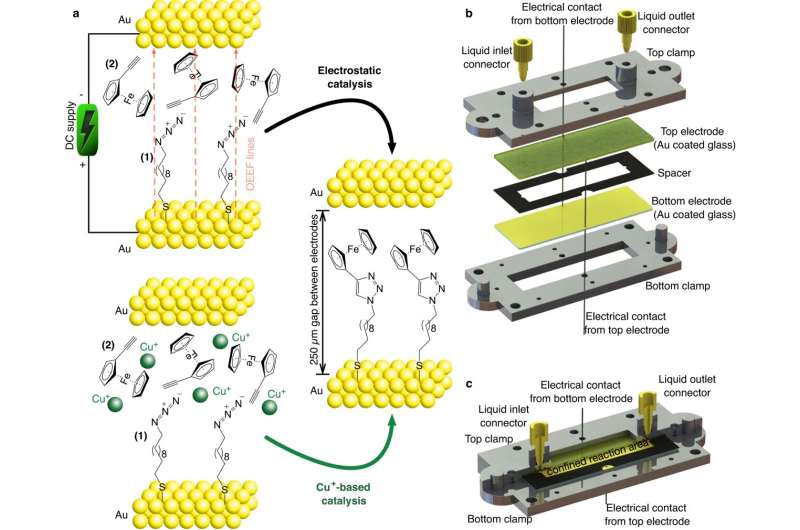
A new, greener, and cheaper method to accelerate chemical reactions has been developed by scientists at King's College London in collaboration with the University of Barcelona and ETH Zurich. Instead of using polluting and expensive metal-based catalysts, the team proved that electric fields can catalyze reactions to produce chemical compounds.
Building on previous research published in 2016 that proved an electric field could catalyze reactions in a nanoscale gap, the scientists from the Department of Chemistry upscaled the methodology to catalyze reactions on a centimeter area electrode electrically. This opens the way for a cleaner alternative to metal-based catalysts, potentially transforming the way chemical compounds are produced.
Accelerating chemical reactions between molecules through catalysis is fundamental to making the new materials required by a range of industries and technologies that produce high-value-added products such as pharmaceuticals. Currently, these reactions mainly rely on catalysts that contain precious metals such as platinum, palladium, and rhodium.
Not only are these highly expensive and environmentally damaging to extract, but the practice uses large amounts of energy, and the toxic by-products are difficult and costly to dispose of.
Enabling catalysis through electric fields enables a cheaper, more energy-efficient, and less pollutant solution to this problem.
"Using electric fields as the only catalyst for chemical reactions has long been theoretically predicted. The idea came from scientists studying the mechanisms of enzymatic catalysis in nature, who predicted that large electric fields within the enzyme's active sites could be acting as a catalyst in enzymatic chemical reactions," says Professor Ismael Diez Perez.
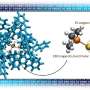
"In 2016 at King's, we actually proved this theory in the lab. By exposing two reactants to a voltage-biased nanoscale gap, we created a force field that accelerates the formation of the reaction product."
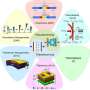
"Since then, we've developed a new technology to enable electrified chemical production on a far larger scale. We designed a microfluidic cell that creates a continuous flow of reactants that are catalyzed together within an electric field. The interface of the electrodes confined within the microfluidic channel induces a chemical reaction on centimeter areas of the electrodes, producing new chemical compounds."
Professor Diez Perez and his team believe this process could transform the pharmacological industry, which relies on the production of very fine, value-added chemical compounds, normally highly expensive to produce through traditional catalysis methods. The scientists even predict the electric field could be controlled to form pure isomeric chemical compounds—a vital step in producing drugs that bear the appropriate molecular shape for the human body to recognize.
"This breakthrough signals the start of a very exciting step change in transforming the way we manage catalysis. We have now proven that electrical fields can be scaled up to produce milligrams of chemical compounds, the next step is to build even larger models for use across many different fields and industries—enabling cheaper, greener production methods," says Deiz Perez.
The work is published in the journal Nature Communications.
More information: Semih Sevim et al, Electrostatic catalysis of a click reaction in a microfluidic cell, Nature Communications (2024). DOI: 10.1038/s41467-024-44716-2
Journal information: Nature Communications
Provided by King's College London