This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop new class of catalysts for green production of fine chemicals and pharmaceuticals

A research team has developed a new class of catalysts—known as heterogeneous geminal atom catalysts (GACs)—that promotes greener and more sustainable manufacturing processes for fine chemicals and pharmaceuticals.
Fine chemical and pharmaceutical manufacturing are major sources of air pollution, with recent studies showing the carbon footprint of the pharmaceutical industry to be heavier than the automotive industry. Beyond greenhouse gas emissions, the pharmaceutical industry is also responsible for other serious environmental impacts, such as water pollution from wastewater released by manufacturers.
"Developing alternative catalytic systems capable of achieving atomic-level precision while ensuring recoverability is at the forefront of our mission to revolutionize sustainable manufacturing processes for fine chemicals and pharmaceuticals. This ground-breaking achievement is the outcome of a close collaboration between several institutions," said Associate Professor Lu Jiong from the Department of Chemistry under the NUS Faculty of Science, who led the team of NUS researchers.
The study was a collaboration involving Associate Professor Koh Ming Joo and Assistant Professor Zhu Ye from the Department of Chemistry under the NUS Faculty of Science, Professor Li Jun from Tsinghua University in China, Professor Javier Pérez-Ramírez from ETH Zurich in Switzerland and Dr. Xi Shibo from the Agency for Science, Technology and Research (A*STAR) in Singapore. The research was published in the journal Nature on 20 September 2023.
Developing a new class of catalyst
The synthesis of organic compounds requires a series of steps known as transition metal-catalyzed coupling reactions. These chemical reactions are indispensable for forming essential chemical bonds during the synthesis of a chemical compound. However, catalysts that are currently used in these reactions pose a number of challenges, such as high production cost, difficulty in catalyst separation for recovery and reuse, and metal contamination which is harmful to the environment. The structural architecture of current catalysts also limits their capacity to carry out complex reactions.
NUS researchers, together with their international collaborators, developed a new class of GACs to circumvent these challenges and boost the potential for more sustainable and environmentally friendly pharmaceutical manufacturing processes.
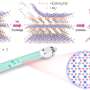
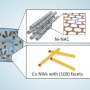
One key feature of this new class of catalyst is the presence of two metal cores made up of copper ions that allows for more efficient and selective reactions. The research team used a material called polymeric carbon nitride (PCN) to act as a supporting structure to hold the two copper ions for them to work together in the chemical reactions. The researchers tinkered with the structure to discover that approximately 0.4 nanometers was the perfect distance between these two copper ions for them to function as one unit to carry out important chemical reactions.
The novel catalyst takes on a unique heptazine chain structure that allows it to be dynamic and adaptable during chemical reactions for the two copper ions to efficiently bring two reactants together for a chemical bond to form; such a chemical reaction is known as cross-coupling. This structure also reduces the minimum amount of energy needed for a chemical reaction to occur.
The research team then tested the newly developed catalyst in some chemical reactions involved in creating commonly used drugs and chemical compounds to demonstrate its efficiency compared to conventional catalysts, and researchers also quantified the environmental benefits of this new type of GACs.
Novel GACs for greener chemical processes
To showcase the versatility of the newly developed GACs, the researchers assessed its performance in various chemical reactions, such as the formation of multifunctional heterocyclic compounds that are commonly used in the production of pharmaceuticals.
The team also reported that the new catalyst can improve the yield of the final product. For example, using the novel GACs, more bromide substrates were readily available to successfully improve the yield of dutasteride, which is used primarily to treat prostate disease, from 53% to 62%, compared to using conventional metal catalysts.
The researchers put the catalyst through nine consecutive cycles of chemical reactions, and discovered that it could remain stable with no detectable loss of copper ions from the original structure. This means that the amount of waste and risk of metal contamination can be significantly reduced.
In addition, the new GACs can be readily recovered and reused, underscoring its potential in boosting sustainability in the chemical and pharmaceutical industries.
The researchers also analyzed the environmental benefits of using the novel catalyst in chemical reactions and found that it achieves a carbon footprint that is 10 times lower than using conventional catalysts.
By outperforming conventional catalysts through increased yield, higher efficiency and improved environmental impact in cross-coupling reactions, this new class of GACs are an attractive option for adoption in the fine chemical and pharmaceutical industries.
"Our goal in the near future is to create a library of GACs by carefully adjusting the specific types and combinations of geminal metal centers. This can potentially transform the conventional methods of chemical production. It could signify the dawn of a new era where GACs play a pivotal role in achieving greener and more environmentally friendly chemical and pharmaceutical manufacturing," added Assoc Prof Lu.
More information: Xiao Hai et al, Geminal-atom catalysis for cross-coupling, Nature (2023). DOI: 10.1038/s41586-023-06529-z
Journal information: Nature
Provided by National University of Singapore