This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
How cells manage their mRNA stockpile and its output
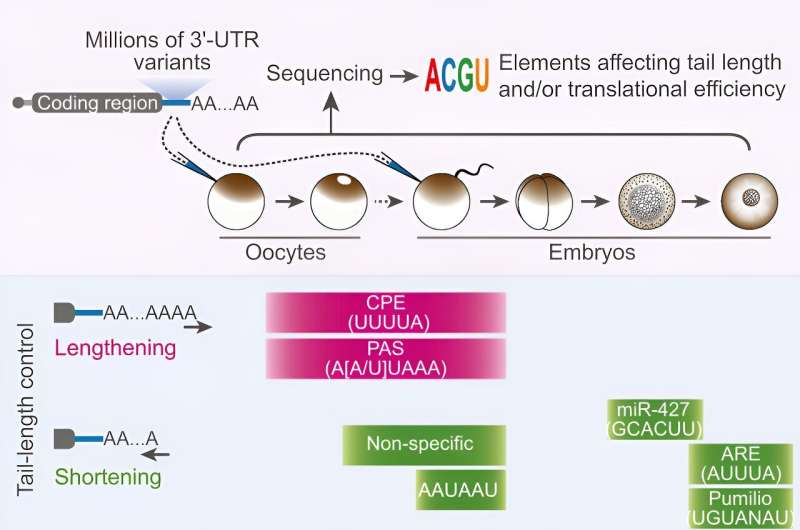
In a typical cell, genes encoded in DNA are used to make messenger RNA (mRNA), which is used to make proteins, and this process of gene expression keeps the cell running. Gene expression is regulated in each cell such that specific genes are turned on (making proteins) and their output is dialed up or down, driving the identity and behavior of each cell.
However, there is an unusual set of cells in which gene expression cannot follow its typical pattern: developing eggs and the embryos into which they may grow. In these cells, there is a brief period before the embryo's genome is ready to be expressed when no new mRNAs are made from genes. The only genetic material that the cells have to work with is a stockpile of mRNAs previously made from the maternal genome.
With no ability to make new mRNA, cells must instead regulate gene expression by adjusting how much protein is made from each of the stockpiled mRNAs at any point in time. They must also carefully time the removal of the maternal mRNAs. If the cells degrade the maternal RNAs too early, there are no backup copies available. If they degrade the maternal RNAs too late, that may interfere with the handoff in which the embryo's genome takes over from the maternal genome.
Due to this unique situation, developing eggs and embryos have evolved a unique system to regulate their mRNAs. In research published in Developmental Cell on March 8, Whitehead Institute Member David Bartel, also a professor of biology at the Massachusetts Institute of Technology and a Howard Hughes Medical Institute Investigator, and postdoc in his lab Kehui Xiang detail the intricate regulatory system that these cells use to manage their mRNA stockpile and its output.
Tail length determines RNA output, but what determines tail length?
Each mRNA has a tail made up of a string of adenosines or "A"s, one of the building blocks or bases of RNA. This is called its poly(A) tail. Researchers knew that in developing eggs and early embryos, an mRNA's efficiency, or the rate at which it is used to make protein, is based on the length of its tail; the longer the tail, the more protein will be produced. (Outside this period of early development, the poly(A) tail plays a different role.)
Since tail length determines an mRNA's efficiency, what Bartel and Xiang wanted to find out was what regulates the length of an mRNA's tail. Whatever regulates tail length is ultimately regulating gene expression in these cells.
Xiang and Bartel began their search by looking at a region within each mRNA that contains non-coding or untranslated RNA. Every mRNA has untranslated regions, and these can contain different regulatory sequences that affect how other molecules interact with the mRNA.
Researchers knew that the 3' untranslated region of mRNAs contains two sequences that are required for an RNA's tail to be lengthened when it is outside of the nucleus: a sequence called the cytoplasmic polyadenylation element (CPE) and one called the polyadenylation signal (PAS).
The CPE and PAS are each binding sites, with sequences that match specific proteins. The matching proteins bind to the two sites, and together with other proteins form the machinery that leads to tail lengthening.
Researchers knew the sequence of the PAS, but the correct identity of the CPE had been elusive. Various sequences had been proposed, but many mRNAs that undergo tail lengthening contained none of the suggested CPEs.
Xiang and Bartel used a systematic approach to identify the CPE of frogs. They created a library of millions of mRNAs, each with different sequences in the 3' untranslated region. Then they looked at what happened to the tail length of these mRNAs in the developing eggs of frogs. Ultimately, this allowed them to narrow in on the CPE: UUUUA, a sequence of five bases.
They found evidence that this CPE has been conserved in evolution and is shared by mice and humans. They also found that fish embryos have a slightly different CPE, in which the last base of the sequence can be either an A or a U.
Having identified the CPE, the researchers could then experiment to determine what modifies the CPE's effect on tail length. They found that the bases to either side of the CPE can strengthen its effect—this may explain some of the previously suggested CPEs, which tended to be longer sequences. Likewise, the bases closest to the PAS can modify its effect.
Other modifying factors included how many copies of the CPE and PAS were present in an mRNA, how close the CPE was to the PAS, and how close the PAS was to the tail of the mRNA. The combination of these factors determines how long an mRNA's tail will get, leading to highly individualized tail lengths across the mRNAs found in developing eggs and embryos.
"There's a very complex tapestry of tail-length changes that is normally occurring in early development, and it had been a mystery as to how that was occurring. Now we have a sense of why different mRNAs behave so differently from each other," Bartel says.
Other mRNA regulators in early development
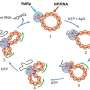
Bartel and Xiang found that while only a few sequences affect tail lengthening, a variety of sequences are involved in tail shortening in developing eggs and early embryos. Different regulators affect different mRNAs, so the cells can repress the maternal mRNAs in targeted waves.
The tails of many mRNAs are shortened in an initial large wave, and then smaller waves shorten tails in a more individualized manner, allowing careful orchestration of the switch away from maternal genome control.
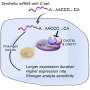
The researchers also identified ways in which the cells can repress mRNAs independent of tail length. If an mRNA contains stretches of cytosine or "C" bases in the 3' untranslated region, this represses the mRNA. Also, when immature egg cells are dormant before their final maturation, containing a CPE represses mRNA.
This finding is consistent with previous research, and makes sense given that many of the genes that Bartel and Xiang identified as containing CPEs are related to cell division. These genes need to be off during dormancy, and then they need to be very active during egg and embryonic development. With its dual roles, the CPE works as an elegant switch between these states.
Xiang and Bartel's findings paint a picture of an intricate system of regulation for mRNA during early development. This enables precise tuning, such that the output of each mRNA can be dialed to the right level at each stage of development. This work sheds light on how a single fertilized egg cell begins the incredible process of becoming an entire new organism.
Follow-up work may shed further light on how these mechanisms affect fertility. Xiang and Bartel are using their findings to create algorithms that can predict a given mRNA's tail length. The researchers plan to use their predictive tools to gain insights into female infertility. For example, how do mutations to genetic sequences involved in lengthening mRNA tails affect an egg or embryo's viability?
"Not a lot of attention has been paid to mutations in the 3' untranslated region because it's a non-coding region, but we're hoping with more genomic data and our improved modeling, we can pinpoint human genetic variants that could potentially have an impact on fertility. That's a big part of our motivation," Xiang says.
More information: Kehui Xiang et al, Control of poly(A)-tail length and translation in vertebrate oocytes and early embryos, Developmental Cell (2024). DOI: 10.1016/j.devcel.2024.02.007
Provided by Whitehead Institute for Biomedical Research