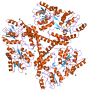This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Novel siRNA backbone enhances stability, durability of oligonucleotide therapeutic platform
A biochemical breakthrough using simple carbon atoms, by Ken Yamada, Ph.D., and Anastasia Khvorova, Ph.D., has dramatically improved the stability and efficacy of a potential oligonucleotide therapeutic platform in mice. This discovery, published in Nature Biotechnology, has the potential to improve the durability of oligonucleotide drugs, including small interfering RNAs, and to bring these therapies to cell types outside of the liver for the first time.
"The innovation of our study is a modification to the chemical composition behind the oligonucleotide platform's architecture," said Dr. Khvorova, the Remondi Family Chair in Biomedical Research and professor of RNA therapeutics.
"Using a relatively simple change—extending the RNA backbone with single carbon atoms—we've been able to significantly improve the duration that oligonucleotides persist in cells by effectively hiding them from the nucleases that normally break them up. This type of modification is critical to the success of oligonucleotide drugs as a class."
Oligonucleotide drugs, including siRNAs, are a new class of medicine that enables modulation of disease-causing genes for therapeutic effect.
There are six siRNAs approved for clinical use by the Food and Drug Administration—all in the liver—with more in late-stage clinical trials. The first of which, patisiran, now sold under the brand name Onpattro, was approved in 2018 for people with hereditary transthyretin-mediated amyloidosis, a rare and fatal liver disease.
Oligonucleotides, however, are very unstable in their natural state. The enzymes responsible for breaking down oligonucleotide molecules, called nucleases, act very quickly inside living cells, giving the drugs a limited amount of time to achieve a therapeutic effect.
As a result, scientists have been challenged to design chemical scaffolds that can stabilize, sustain and extend the longevity of oligonucleotide drugs necessary to achieve therapeutic success in living tissue, especially cells other than liver cells.
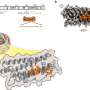
Most oligonucleotide modifications focus on changing the phosphorothioates or sugar molecules existing in the chemical backbone. Dr. Yamada and Khvorova, however, took a different approach, homing in on the carbon molecules in the backbone's chemical structure.
"In nature, a single or a few methylations to nucleobases and amino acids have been shown to slightly alter the structure of DNA, RNA and proteins that profoundly impacts in-cell gene expression, small structural change making a big impact in the cell," said Yamada, assistant professor of RNA therapeutics.
"This process inspired us to apply an analogous approach to the oligonucleotide platform's backbone—a small structural change making a big impact on siRNA's durability by increased stability and enhanced efficacy."
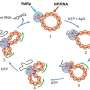
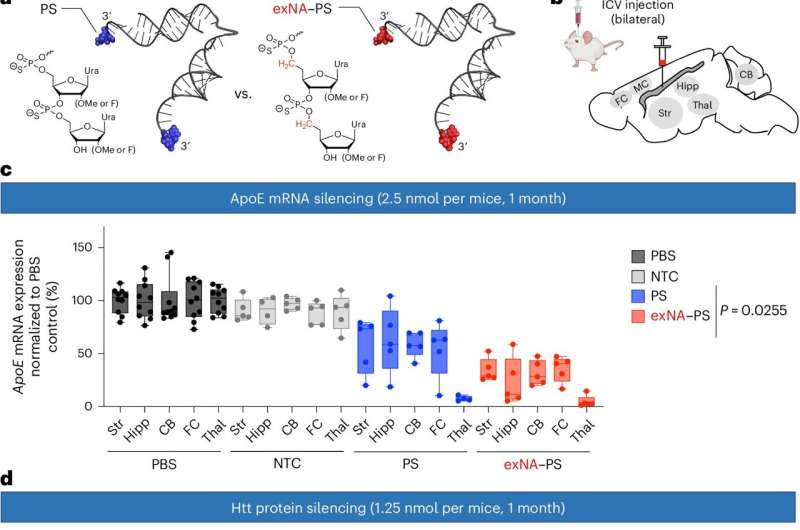
Testing several permutations, Yamada and Khvorova found that an extra carbon atom inserted in the correct spot along the nucleoside effectively hid the oligonucleotide molecules from degradation by the nuclease enzyme. This resulted in a more stable and efficient oligonucleotide, which was dubbed extended nucleic acid or exNA, that persisted 32-fold longer than its phosphorothioate-modified counterparts.
"It's all about where and how you add the carbon," said Yamada. "Even a slight structural modulation can have a huge impact on efficacy if you do it in the right place and in the right way."
Yamada added, "If we could garner even a 10-fold increase in persistence, we could reduce dosage or clinical treatment time from once every two weeks to every five months. To have a 32-fold increase in mice models is tremendously encouraging."
Khvorova said, "Muscle, kidney, central nervous system, pancreas, eye—a host of tissues and diseases that were beyond our capabilities are now potentially feasible drug targets for oligonucleotide therapies."
The next step for researchers going forward is to begin testing the safety and longevity of their oligonucleotide backbone in clinical trials.
More information: Ken Yamada et al, Enhancing siRNA efficacy in vivo with extended nucleic acid backbones, Nature Biotechnology (2024). DOI: 10.1038/s41587-024-02336-7
Journal information: Nature Biotechnology
Provided by University of Massachusetts Medical School