This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New technique developed for targeted protein degradation
A new study published in Nature Communications from researchers at Karolinska Institutet solves a long-standing problem by establishing a system that allows site-specific protein degradation within mitochondria, the cellular hubs for energy production and metabolism.
Understanding how cells work often requires manipulating protein function. Methods used to do this usually cause total ablation of protein function and cannot provide information about their specific roles within different cellular compartments. This is especially challenging for organelles like mitochondria.
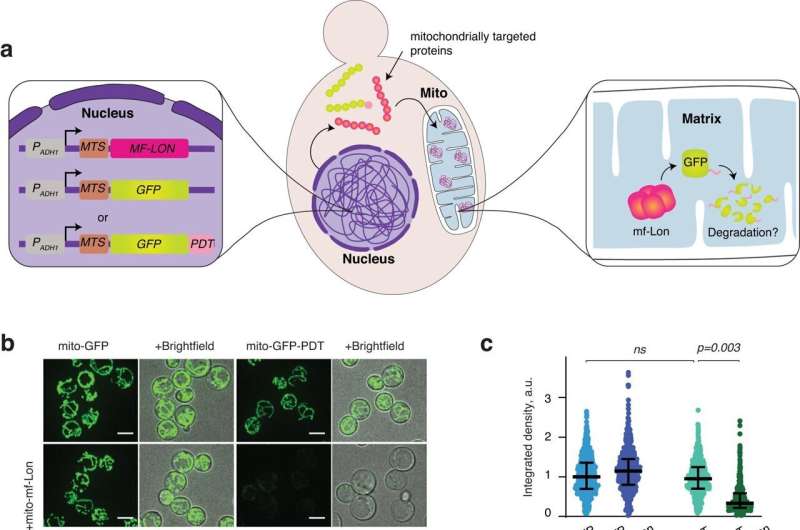
To address this, the researchers present, for the first time, a technique for targeted protein degradation within the mitochondria of yeast and human cells. They have also devised a way to control the induction of degradation, thereby allowing time-resolved analysis.
A crucial aspect of this technique is its ability to disengage mitochondrial function from the rest of the cell. Having bacterial origins, mitochondria possess a certain degree of autonomy, such as their own genome, an intricate system for energy production, and the capability to replicate.
Despite this, proper mitochondrial function heavily relies on other organelles, including the nucleus. When the crosstalk between mitochondria and other organelles fails, mitochondrial dysfunction ensues, which is strongly linked to neurodegenerative diseases and age-related disorders like cancer.
Unsurprisingly, the specific targeting of mitochondrial function is therefore essential to understand—and eventually treat—such disorders.
"We introduced a bacterial protease inside yeast and human mitochondria to achieve mitochondria-specific protein degradation. We did this with the knowledge that mitochondria are evolutionarily derived from bacteria, and a bacterial protease should thus work in the mitochondrial environment.
"We chose to use the Lon protease from a mollicute, which detects and degrades a protein tag called PDT. This can be added to the protein under investigation by genetical engineering.
"To our great excitement, we found that the Lon protease was specifically able to degrade PDT-tagged proteins within the mitochondria in yeast and human cells," says Camilla Björkegren professor from the Department of Cell and Molecular Biology.
Swastika Sanyal, who developed the system, says that the plan is to go further with this project. "Our next step will be to use the PDT-tagging system we have developed to identify the mitochondria-specific functions of DNA-maintaining enzymes. Most of these proteins also maintain nuclear DNA, so by degrading them within mitochondria only, we can discern their direct role in mtDNA maintenance.
"These studies will also enable us to better understand what goes wrong during mitochondrial dysfunction, which can result from mutated and deleted mtDNA."
More information: Swastika Sanyal et al, A system for inducible mitochondria-specific protein degradation in vivo, Nature Communications (2024). DOI: 10.1038/s41467-024-45819-6
Provided by Karolinska Institutet