This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Molecular mechanism of transmembrane bilirubin transport by human ABCC2 transporter revealed
The metabolic process of bilirubin has been a focus of medical research since the abnormal accumulation of bilirubin has been found to be associated with a variety of diseases. Bilirubin is a substance produced by the breakdown of aging or damaged red blood cells, and its effective removal is essential for human health.
A research team led by Prof. Chen Yuxing and Prof. Zhou Congzhao from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences has revealed the three-dimensional structure and working mechanism of the human bilirubin transporter ABCC2. The study was published in Nature Communications.
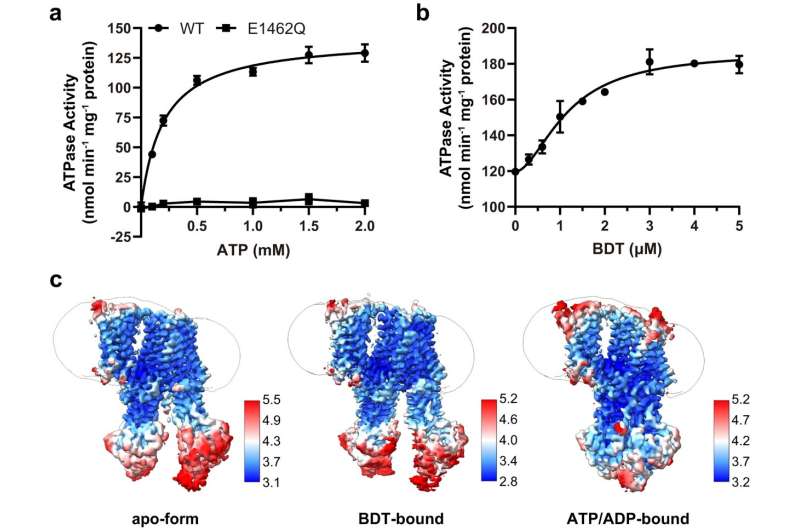
The researchers analyzed the structure of the ABCC2 protein determined by single-particle cryogenic electron microscopy (cryo-EM) in three different forms: the apo-form, the substrate-bound form, and the ATP/ADP-bound structures. They proposed a unique regulatory domain (R domain) that precisely controls ABCC2 substrate recognition and transport.
The R domain was folded into a hairpin structure that helped the ABCC2 protein stay still before it encountered bilirubin. But when it bound to a physiological substrate analog, bilirubin ditaurate (BDT), the hairpin part moved away. Additionally, the R domain helped proteins select high-affinity conjugated bilirubin for preferential outward transport. The protein then changed its conformation in response to the hydrolysis of ATP, releasing the substrate into the duct.
In particular, the researchers pointed to a specific ABCC2 mutant (R1150H) that weakens the function of the R domain and leads to decreased transport activity, which explains the molecular mechanism of diseases such as Durbin-Johnson syndrome.
This study identified the key role of ABCC2 protein in the treatment of bilirubin under the fine regulation of the R domain, providing a new perspective for understanding bilirubin metabolism and a possibility for targeted treatment of the related genetic diseases.
More information: Yao-Xu Mao et al, Transport mechanism of human bilirubin transporter ABCC2 tuned by the inter-module regulatory domain, Nature Communications (2024). DOI: 10.1038/s41467-024-45337-5
Provided by University of Science and Technology of China