This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers determine how type II restriction endonuclease Sau3AI cleaves DNA
Sau3AI is a type II restriction enzyme widely used for genetic manipulation, such as genome library construction. Sau3AI consists of two domains, the N-terminal domain (Sau3AI-N) and the C-terminal domain (Sau3AI-C). How these two domains work together to cut DNA remains unclear.
Recently, a research team led by Prof. Yu Feng from the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences and Prof. He Jianhua from Wuhan University reported a self-activating mechanism in which the Sau3AI C-terminal domain opens the N-terminal catalytic domain through allosteric effects to achieve cleavage of DNA-specific sites. The results were published in Structure on Aug. 30.
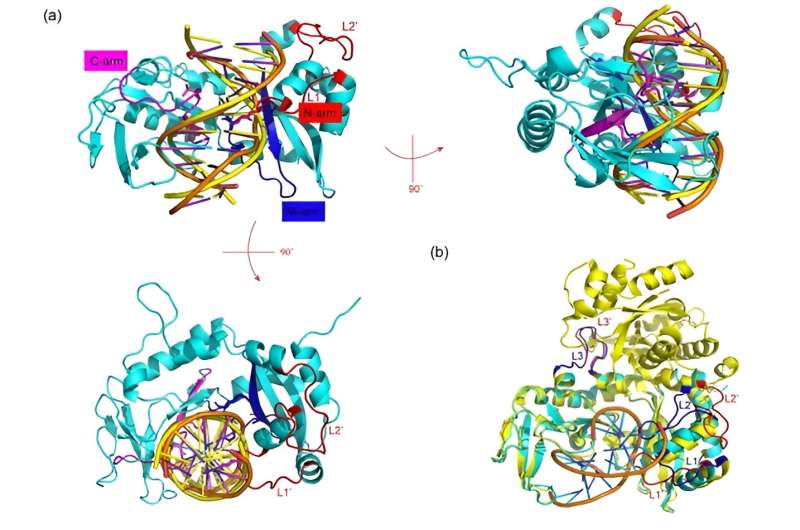
Due to DNA cleavage activity, Sau3AI cannot be expressed in Escherichia coli, and thus, a catalytic site mutant, Sau3AI-E64A, was used for exogenous expression and structural research. The crystal structure of the Sau3AI-E64A mutant reveals that when DNA is not bound, a loop region (333–342) of the C-terminal domain hangs over DNA binding site of N-terminal domain, preventing N-terminal domain from binding to DNA.
Analysis of the Sau3AI C-terminal domain and DNA complex structure indicated that the loop region (261–268 and 280–295) of C-terminal domain was significantly altered after binding to DNA, implying that Sau3AI may undergo conformational changes after C-terminal domain binds to DNA.
Furthermore, the researchers conducted gel shift experiment on the DNA binding key amino acid residue mutants (K257A, S424A, and T435A) in C-terminal domain to confirm that C-terminal binding DNA plays a crucial role in activating the catalytic activity of N-terminal domain. These results suggest that C-terminal domain activates the enzymatic activity of N-terminal domain through allosteric effects.
The researchers suggested that Sau3AI is a type IIE restriction enzyme, but unlike other type IIE restriction enzymes, it is monomeric rather than homodimer. Two different domains in Sau3AI play a similar role to homodimers in other type IIE restriction enzymes, suggesting that Sau3AI represents a new subclass of type IIE restriction enzymes.
More information: Yahui Liu et al, The crystal structures of Sau3AI with and without bound DNA suggest a self-activation-based DNA cleavage mechanism, Structure (2023). DOI: 10.1016/j.str.2023.08.005
Journal information: Structure
Provided by Chinese Academy of Sciences