This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
AI-assisted robot lab develops new catalysts to synthesize methanol from CO₂

Artificial intelligence and automated laboratory infrastructure are massively accelerating the development of new chemical catalysts. With these tools, researchers at ETH Zurich are developing catalysts for efficiently and cost-effectively synthesizing the energy source methanol from CO2.
Catalysts are chemistry's hard-working little helpers. They accelerate reactions and reduce the energy required for a reaction to take place. The more specific and effective a catalyst is, the more effectively any undesirable side reactions are suppressed.
In nature, enzymes have the job of specifically boosting the required metabolic processes from among the almost infinite reaction possibilities of the chemical soup within cells. In chemical plants, metal catalysts are usually employed to increase product yield.
The researchers working on the Swiss Cat+ technology platform at ETH Zurich, led by Paco Laveille, have now developed a fully digitalized and automated method that enables them to find new and better metal catalysts much faster than before. Their process consists of a combination of artificial intelligence (AI) for calculating promising catalyst compositions and an automated synthesis and test laboratory.
With this infrastructure, it took the team less than six weeks to successfully develop about 150 catalysts compositions for producing methanol from CO2. The best catalysts are cost-effective and exhibit high conversion rates with a low proportion of byproducts. "This new method saves a huge amount of time," Laveille says. "With a conventional approach, our experiments would have taken years."
The researchers have published two papers on their method. The first was published last year in CHIMIA and the second this week in Chem Catalysis.
Methanol is regarded as one of the key elements for a sustainable hydrocarbon economy. A close chemical relative of ethanol (i.e., drinking alcohol), the substance can be used both as a fuel and as a raw material for the production of organic compounds such as medicines, plastics or paints.
Because it is a liquid, methanol is much easier to transport and store than gaseous hydrogen and methane, two other sources of energy. What's more, using methanol in the existing supply infrastructure and engines of today's petrol technology requires only minor modifications.
Narrowing down the possibilities through clever preselection
In the search for optimum catalysts for methanol production, there's one big problem: Theoretically, atoms can be combined in an almost infinite number of ways to form a catalyst. "The chemical space in which we're searching for catalysts comprises around 1020 possibilities—that's one hundred billion billion. So we're literally looking for a needle in the chemical haystack," explains Christophe Copéret, a professor at the Laboratory of Inorganic Chemistry at ETH Zurich and co-initiator of the Swiss Cat+ project.
To narrow down the huge range of possibilities, the researchers made a preselection based on experience and economic requirements. A catalyst that can be used on a large scale needs to be not only effective but also inexpensive. For that reason, the main active ingredients for the catalyst were limited to three comparatively cheap metals: iron, copper and cobalt.
In addition to these main metals, the researchers considered three elements that are traditionally added to catalysts in small quantities for the purposes of doping, as well as potassium, which is also contained in many catalysts. As to carrier materials, the researchers limited themselves to four typical metal oxides. Multiplied by the different mixing ratios, this still resulted in 20 million possible combinations.

Taking iterative steps with AI-supported statistics
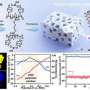
At this point, the researchers brought an AI algorithm into play that uses what is known as Bayesian optimization to find the best possible solutions. This special form of statistics is particularly suitable when only a small amount of data is available. Unlike in classical statistics, the probability doesn't derive from the relative frequency as calculated from numerous experiments. Instead, the calculation takes into account the probability that can be expected based on the current state of knowledge.
In the initial round, the algorithm randomly selected 24 catalyst compositions that met the specifications drawn up for the purposes of limiting the complexity. These catalysts were produced directly using the Swiss Cat+ automated laboratory infrastructure and then tested.
Delivering lots of highly reliable results quickly
The results of this initial selection served the researchers as the starting point for an AI prediction; the catalyst compositions thus predicted were in turn automatically synthesized and tested. For this first demonstration test, the scientists had their integrated system complete a total of six such rounds.
The fact that the results improved between rounds not in a linear fashion, but rather by leaps and bounds, was entirely intentional: Not only does the algorithm optimize the results of earlier rounds, it also includes an exploratory component that feeds completely new compositions into each round and learns about the chemical space. This is how the researchers prevented the calculations from getting stuck in an optimization dead end among all the possibilities.
Generating data beyond petrochemicals
In this first project, though, the researchers' primary concern wasn't to come up with the best possible catalyst for methanol synthesis. "At present, knowledge about catalysts for fuel production is based predominantly on expertise from the oil industry," Copéret says. "When it comes to reactions for use in the sustainable energy industry, reliable data is still largely lacking."
However, AI algorithms and human research intelligence need that data before they can search in a more targeted way in the vast space of chemical possibilities. "And that's precisely the kind of high-quality, reproducible data our AI-assisted robot laboratory now delivers. It's certain to take catalyst research a long way forward," Laveille adds.
More information: Paco Laveille et al, Swiss CAT+, a Data-driven Infrastructure for Accelerated Catalysts Discovery and Optimization, CHIMIA (2023). DOI: 10.2533/chimia.2023.154
Adrian Ramirez et al, Accelerated exploration of heterogeneous CO2 hydrogenation catalysts by Bayesian-optimized high-throughput and automated experimentation, Chem Catalysis (2024). DOI: 10.1016/j.checat.2023.100888
Provided by ETH Zurich