This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Unveiling sodium channel dynamics: New insights into cell biology using high-speed atomic force microscopy
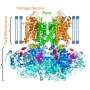
The transport of ions to and from a cell is controlled by pore-forming proteins embedded in the cell membrane. In particular, voltage-gated sodium channels (VGSCs) govern the transfer of sodium (Na+) ions, and play an important role in the regulation of the membrane potential—the voltage difference between the cell's exterior and interior.
In electrically excitable cells such as neurons and muscle cells, VGSCs participate in the generation of action potentials; these are rapid changes in the membrane potential enabling the transmission of, for example, neural signals. The precise structural changes occurring in VGSCs are not completely understood, however.
Now, Ayumi Sumino and Takashi Sumikama from WPI-NanoLSI, Kanazawa University in collaboration with Katsumasa Irie from Wakayama Medical University and colleagues have succeeded in observing the structural dynamics of VGSC by means of high-speed atomic force microscopy (HS-AFM), a method capable of imaging the nanostructure and subsecond dynamics of biomolecules.
The work is published in the journal Nature Communications.
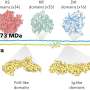
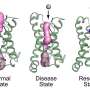
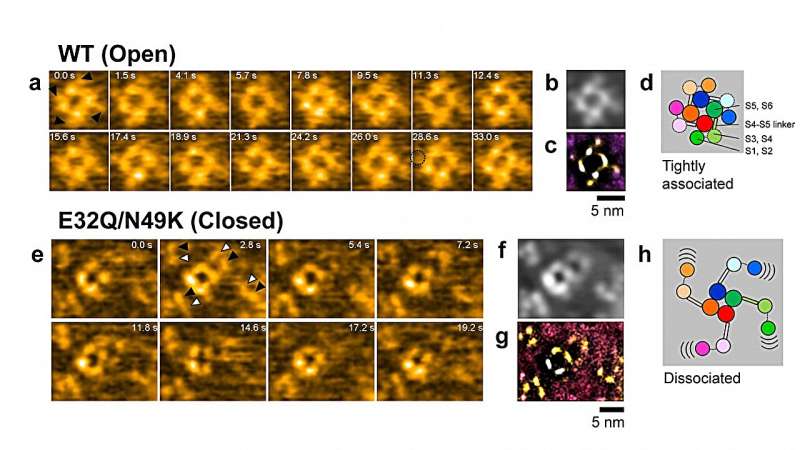
VGSCs can be in three different states—resting, inactive and active. In the latter state, Na+ ions can pass through the channel; in the resting and inactive states, which are structurally different, ions cannot pass. The basic structure of a VGSC consists of two modules: Voltage sensor domains and pore domains. These domains form a square arrangement, with the ion pore at its center. An important open question is whether the voltage sensor domains dissociate from the pore domains when the channel closes.
Sumino and colleagues performed experiments on three VGSCs. One is the sodium channel of a particular bacterium (Arcobacter butzleri), the other two are mutants of this bacteria. These three VGSCs have different voltage dependencies, with activation voltages starting at -120 mV, -50 mV and 0 mV, so that at the experimental conditions (0 mV), the VGSCs are in different states.
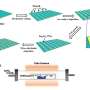
In order to provide insights into the structural dynamics of these three VGSCs, the researchers applied HS-AFM, a powerful technique for producing image sequences of biochemical compounds. A single AFM image is generated by laterally moving a tip just above the sample's surface.
During this xy-scanning motion, the tip's position in the direction perpendicular to the xy-plane (the z-coordinate) will follow the sample's height profile. The variation of the z-coordinate of the tip then produces a height map—the image of the sample. The generation of such AFM images in rapid succession then produces a video recording of the sample.
The HS-AFM results revealed that for the mutant VGSC in the resting state, the voltage sensor domains are indeed dissociated from the pore domains. Furthermore, the researchers found that the dissociated voltage sensor domains of neighboring channels connect to form pairs—this is referred to as dimerization.
The observation of the dissociation of voltage sensor domains, as well as the dimerization between pore channels, are findings that will lead to a better understanding of what causes pores to close in the resting state, and how the development of action potentials is regulated. Quoting the scientists, dimerization offers "a potential explanation for the facilitation of positive cooperativity of channel activity in the rising phase of the action potential."
More information: Ayumi Sumino et al, Voltage sensors of a Na+ channel dissociate from the pore domain and form inter-channel dimers in the resting state, Nature Communications (2023). DOI: 10.1038/s41467-023-43347-3
Journal information: Nature Communications
Provided by Kanazawa University