Researchers discover non-canonical ion channel activation pathway
The passage of ions through the cell membrane is controlled by ion channels, which are protein complexes that regulate vital processes, such as the heartbeat, and are a target of drug development. Now, a study at the University of Wisconsin, led by a Spanish researcher, presents a novel model to explain how the pores of these channels open and close.
Ion channels confer electrical and excitable capacities upon the cells, and are responsible for important cellular processes such as the generation and propagation of the heartbeat, the signal transmission between neurons, the secretion of neurotransmitters, and the diffusion of pain through the body. When ion channels fail, such problems as cardiac or neuronal pathologies occur.
"Due to their nature and function, they are magnificent therapeutic targets for drugs fighting high blood pressure, arrhythmias and other diseases, as well as the place where some arachnid or snake toxins act," says researcher Ana Fernández Mariño, who, during her stay in the University of Wisconsin (USA), found a new way of explaining how ion channels are activated.
These are transmembrane protein structures that act as a system of gates to regulate the passage of ions—potassium, sodium, calcium, chloride, etc.—through a pore. The pore is opened or closed by the stimuli coming from another region of the channel, called the voltage sensor, which detects changes in the electrical potential of the membrane.
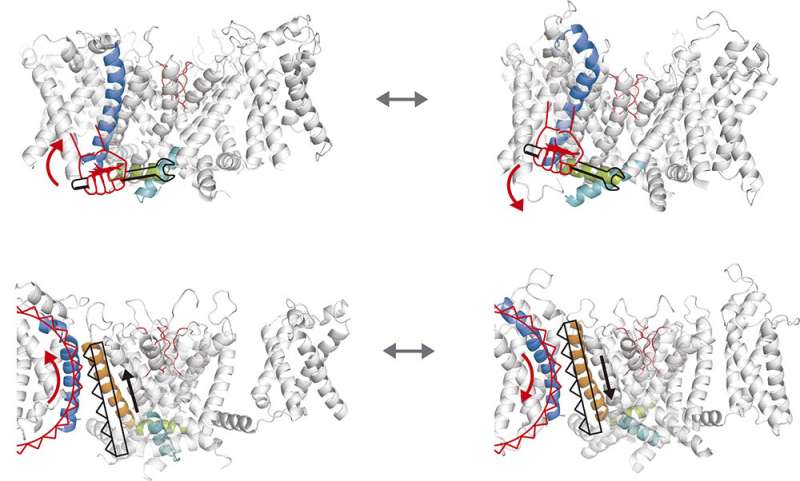
Until now it was thought that the pore and the voltage sensor were coupled through a linker (a helix of about 15 amino acids), which can be triggered by the movement of the voltage sensor. This is the canonical vision of the coupling mechanism between the two parts of the ion channel.
But Fernández Mariño's research has discovered a non-canonical pathway, which involves an amino acid segment made up of part of the voltage sensor and part of the pore. These two segments adjust to each other like a zipper to trigger the opening or closing of the channel. The details are published in Nature Structural & Molecular Biology.
"To carry out the study, we used as a model a potassium channel named Shaker, together with mutagenesis, electrophysiology, fluorescence techniques, molecular dynamics simulations and energy calculations, with which we've been able to analyze the molecular pathways through which the coupling between the voltage sensor and the pore takes place," explains Fernández Mariño.
The new approach helps to explain recent discoveries that some ion channels regulated by voltage such as the so-called human ether à-go-go related gene (hERG) barely have a linker. It is a fragment of only five amino acids and dispensable for the coupling between the two pieces.
"Our study therefore opens the debate on how ion channels in general respond to voltage signals following the non-canonical path, which is valid for both the Shaker type with its well-structured linker and those of the hERG family and others that barely have a linker," Fernández Mariño says. "If we gain a better understanding of how these ion channels work, we will better understand the physiological mechanisms they regulate, as well as their pathologies, and this will help us design new drugs to improve our quality of life."
More information: Ana I. Fernández-Mariño, Tyler J. Harpole, Kevin Oelstrom, Lucie Delemotte and Baron Chanda. "Gating interaction maps reveal a noncanonical electromechanical coupling mode in the Shaker K+ channel". Nature Structural & Molecular Biology 25: 320–326, abril de 2018.
Journal information: Nature Structural & Molecular Biology