This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study identifies reaction conditions that could make autoxidation of aldehydes more environmentally friendly
Approximately 5% of global carbon emissions are attributable to producing the chemicals that are essential to modern life. Creating a sustainable solution to one chemical reaction in particular—the autoxidation of aldehydes—has challenged researchers for decades.
Now, in a study published in Green Chemistry, researchers from Osaka University, Shizuoka Institute of Science & Technology, and collaborating partners have solved this problem. Through reaction kinetics and mathematical modeling, they studied the key details of autoxidation of aldehydes in various solvents, atmospheric conditions, and light sources. The identified environmentally friendly reaction conditions might serve as inspiration for improving other industrially common chemical syntheses.
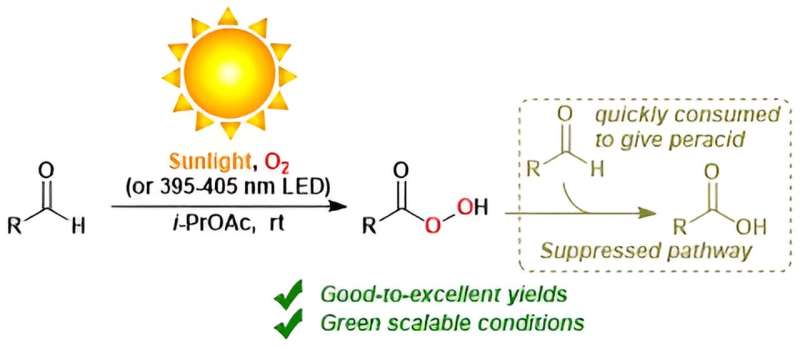
Autoxidation simply refers to oxidation in air at typical temperatures, without a flame or spark input. For example, autoxidation of a common molecule known as an aldehyde produces a molecule known as a peracid.
Peracids are incredibly useful because they can catalyze the conversion of a wide range of molecules by well-established chemistry. Unfortunately, the reaction conditions that are commonly used to produce peracids are wasteful, require dangerous additives, or have other fundamental complications. Optimizing the sustainability of the autoxidation of aldehydes was the goal of the research team's study.
"In our work, we comprehensively study the reaction chemistry that underpins autoxidation of aldehydes into peracids or carboxylic acids," explains Mohamed Salem, lead author of the study. "The experimental setup is simple and suitable for gram-scale syntheses."
The researchers report 18 examples of fast reactions of various aldehydes into peracids, in a pure oxygen atmosphere. These conditions suppressed continued reaction into carboxylic acids.
They furthermore report 32 examples of slow reactions of various aldehydes into carboxylic acids through peracid intermediates, in a normal atmosphere. Some of these 50 reactions proceeded best under sunlight, or alternatively under an LED light.
All reactions were approximately at room temperature, and in safe solvents. This work is the first time that peracids have been synthesized from aldehydes using only sunlight and oxygen.
"We're excited because our research solves a long-standing peracid synthesis problem in green chemistry," says Shinobu Takizawa and Masayuki Kirihara, senior authors. "Our comprehensive studies will help researchers understand the unique chemistry that's imparted by different functional groups in the starting material."
This work is an important step forward in solving a previously vexing problem in peracid synthesis that has limited the environmental sustainability of producing such molecules.
The optimized autoxidation reaction conditions identified in this study are applicable to a wide range of common chemical starting materials. Because the procedures are safe and cheap, practical applications in diverse syntheses should be straightforward.
The research team is currently strengthening its collaboration with MANAC Chemical Partners Co., Ltd. to commercialize these research outcomes.
More information: Mohamed S. H. Salem et al, Light-induced autoxidation of aldehydes to peracids and carboxylic acids, Green Chemistry (2023). DOI: 10.1039/D3GC02951D
Journal information: Green Chemistry
Provided by Osaka University