This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A chemical reaction key to various industries just got greener

From alleviating your allergy symptoms to optimizing herbicide performance, alkylamines are molecules that have many uses. Unfortunately, common methods of producing alkylamines result in harmful waste byproducts. A method of synthesizing alkylamines in a sustainable yet cost-effective way has thus been highly sought after.
Now, in a study published in Green Chemistry, a research team led by Osaka University has found a way. The team has developed a method of alkylamine synthesis that works under mild conditions and produces only water as a byproduct. The simple, environmentally friendly reaction conditions reported here will hopefully inspire advances in other chemical syntheses common in industry.
Sports clothing, furniture, and many other everyday products are produced, in part, by using alkylamines. So how do we produce these wonder molecules?
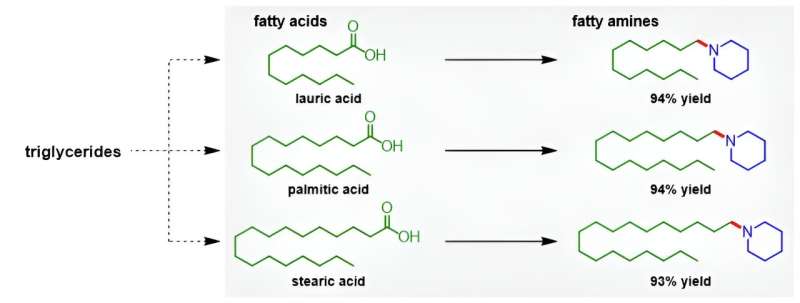
Carboxylic acids are an ideal starting point because they can be sourced sustainably, such as from biomass. However, the synthetic procedures currently used also produce a large quantity of waste or require experimentally difficult reaction conditions, such as high pressures and temperatures. Thus, industry generally avoids carboxylic acids as a starting material for alkylamine production.

Developing a new synthetic protocol that works at experimentally convenient pressures and temperatures was the goal of the research team's study.
"In our work, we unveil a novel catalyst system, a platinum—molybdenum catalyst, that can transform carboxylic acids into amines," explains Katsumasa Sakoda, lead author of the study. "This produces alkylamines, which can be used for surfactants, pharmaceuticals and more."
The researchers' synthetic protocol offers several advantages: one, the reaction conditions are mild, requiring only atmospheric hydrogen pressure and temperatures up to 160°C. Two, the turnover number, i.e., the number of moles of substrate that a mole of catalyst can convert, is high at 363. Three, the catalyst can be reused at least five times. Four, many carboxylic acid and amine starting materials are compatible with the reactions involved, such as converting fatty acids into fatty amines. Five, the reaction yields are high—up to 99%, with water being the only byproduct.
"We're excited because our research improves the environmental sustainability and simplifies the experimental setup of a common class of chemical syntheses," says Tomoo Mizugaki, senior author. "We hope that this is only the first step toward the development of greener catalyst processes."
The team's research is an important step forward in increasing the sustainability of a chemical reaction that is required for synthesizing many everyday products. Because the experimental synthetic procedures are safe and simple, they can be readily used for other catalytic processes.
More information: Katsumasa Sakoda et al, Reductive amination of carboxylic acids under H2 using a heterogeneous Pt–Mo catalyst, Green Chemistry (2023). DOI: 10.1039/D3GC02155F
Journal information: Green Chemistry
Provided by Osaka University