This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
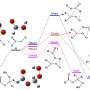
Unimolecular reactions of anti-glycolaldehyde oxide and its reactions with one and two water molecules
Criegee intermediates are produced in the ozonolysis of unsaturated compounds in the atmosphere. These intermediates are especially important because they contribute to the formation of OH radicals during the night and to the formation of secondary organic aerosols. The OH radicals increase the oxidative capacity of the atmosphere, and the aerosols may reflect or absorb sunlight and contribute to cloud formation.
Criegee intermediates also play key roles in the conversion of sulfur dioxide into sulfur trioxide, finally resulting in the formation of sulfuric acid, which is toxic and has been identified as an important nucleation precursor in acid rain mechanisms. Additionally, Criegee intermediates can be a significant sink for atmospheric acids and aldehydes.
We must understand the chemical kinetics of Criegee intermediates, but experimental information is difficult to obtain for these highly reactive intermediates. Therefore, theory can play an important role, especially when carried out at the highest possible levels.
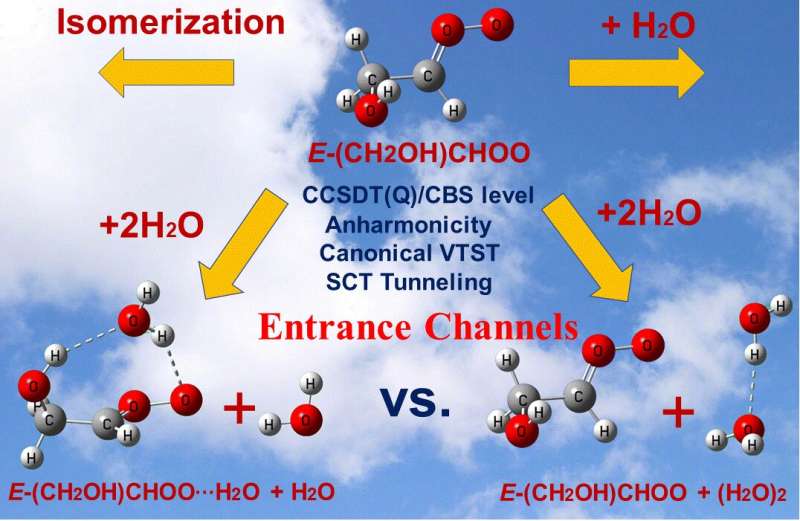
Anti-glycolaldehyde oxide (E-(CH2OH)CHOO) is a substituted Criegee intermediate produced in the ozonolysis of unsaturated alcohols. In a new article published in the journal Research, high-level coupled cluster theory and dual-level multi-structural canonical variational transition state theory with corner-cutting tunneling are used to investigate the unimolecular reaction of ECH2(OH)CHOO and the bimolecular reactions of this intermediate with atmospheric water vapor.
The researchers find that the beyond-CCSD(T) contribution to the electronic structure (i.e., the importance of higher-level correlating excitations) depends on the specific reaction. In addition, reaction with water monomer of the heterodimer of ECH2(OH)CHOO complexed with a water molecule dominates over the reaction of ECH2(OH)CHOO with water dimers at low temperature. This reaction path, although not considered in previous literature, may be common in many other termolecular reactions of Criegee intermediates with two water molecules. This study also finds that the OH substituent on the Criegee intermediate increases its reactivity.
These findings can guide structure–activity relationships currently being used to model atmospheric chemistry, and therefore they may improve our understanding of climate change and acid rain.
More information: Yan Sun et al, Unimolecular Reactions of E -Glycolaldehyde Oxide and Its Reactions with One and Two Water Molecules, Research (2023). DOI: 10.34133/research.0143
Provided by Research