Study sheds new light on production of hydroxyl radicals, which help break down air pollutants
Residents in some areas of the developing world are currently coping with dangerous levels of air pollution. Recent research, co-led by the U.S. Department of Energy's (DOE) Argonne National Laboratory, is leading to a new understanding of a key chemical able to break down some major air pollutants.
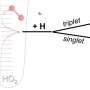
Argonne's Stephen Klippenstein and his collaborators at the University of Pennsylvania examined the Criegee intermediate, a carbonyl oxide that consists of molecules able to break down sulfur dioxide and nitrogen dioxide. Scientists believe these molecules contribute to health issues.
"Astonishingly close agreement of our theoretical work and experimental data is providing important insights into the dynamics of chemical reactions," said Klippenstein.
According to Klippenstein, this research improves models for atmospheric chemistry. The team's work also further validates a major theory for predicting chemical reactivity.
The work allows researchers to understand the dissociation—or the separation of a molecule into atoms—of a prototypical Criegee intermediate in a new way. "This research demonstrates our grasp of tunneling on a molecular system that is of vital importance to the understanding of atmospheric chemistry," said Klippenstein, a distinguished fellow in Argonne's Chemical Sciences and Engineering Division who performed the theoretical calculations.
The researchers showed that quantum mechanical tunneling greatly enhances the production rate of hydroxyl radicals in alkene ozonolysis reactions, which sever multiple bonds under atmospheric conditions.
Hydroxyl radicals are important because of their role in breaking down many pollutants, although in large concentrations, they also contribute to the formation of smog.
The research team, which includes Marsha Lester and Amy Green of the University of Pennsylvania, leveraged results from earlier work. That work showed how combining laser-based experiments with high-level theory, an Argonne hallmark, could allow the researchers to better understand the Criegee intermediate dissociation.
The team succeeded by using deuteration, or the substitution of deuterium atoms for hydrogen atoms, to study how hydroxyl is produced. The chemical properties of deuterium atoms are identical to those of hydrogen atoms, but because they are twice as large in mass, they have a much slower tunneling speed.
The researchers used synthetic chemistry to produce deuterated molecules, which allowed them to substitute the hydrogen atoms in different forms while leaving everything else unchanged.
The team described the results in a recently published paper titled "Selective deuteration illuminates the importance of tunneling in the unimolecular decay of Criegee intermediates to hydroxyl radical products."
More information: Amy M. Green et al. Selective deuteration illuminates the importance of tunneling in the unimolecular decay of Criegee intermediates to hydroxyl radical products, Proceedings of the National Academy of Sciences (2017). DOI: 10.1073/pnas.1715014114
Journal information: Proceedings of the National Academy of Sciences
Provided by Argonne National Laboratory