Visualizing every step of on-surface cycloaddition reactions

By observing individual atoms as they rearrange themselves step by step, chemists at RIKEN have cast new light on the route by which halogenated aromatic molecules join together on a silver surface1. These insights promise to help engineers generate atomically precise nanomaterials and electronic devices.
Molecular electronics and solar cells are two examples of devices that could be made by combining aromatic organic molecules together into extended, π-conjugated structures that conduct electricity.
"A powerful way to directly construct new ring scaffolds with π-conjugated features is the reaction known as the on-surface dehalogenative cycloaddition reaction," says Chi Zhang, a member of Yousoo Kim's Surface and Interface Science Laboratory at RIKEN. "However, a lack of knowledge about the intermediate states that form during this reaction has hindered our ability to precisely design and optimize the reaction products."
A key question is how silver atoms from the surface on which the reaction was conducted interacted with the organic molecules reacting on their surface. Does the silver surface stabilize radical intermediates, or do silver atoms from the surface react to form organometallic intermediates?
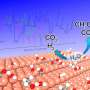
o find out, the team compared the competing reaction mechanisms at play in on-surface dehalogenative cycloaddition reactions by combining high-resolution scanning tunneling microscopy (STM) imaging (Fig. 1) with density functional theory calculations.
"To 'see' and clarify the intermediate states involved in the reaction, we captured the respective intermediate states with well-controlled substrate temperatures," Zhang says. "We then precisely probed them with the STM tip."
By gradually stepping up the temperature and observing the structural changes that occurred at each increment, the researchers were able to study the cycloaddition reaction step by step. They then interpreted the STM images with the help of density functional theory calculations of the structure of predicted reaction intermediates.
The team's combined observations and calculations showed that the formation of organometallic clusters was the dominant reaction pathway over radical formation. In particular, the energetics of the reaction greatly favor participation of a silver atom in the formation of the organometallic dimer over the formation of the diradical intermediate.
"The real-space visualization of the whole reaction processes—combined with further interpretations by theoretical calculations—finally clarified the stepwise metal-mediated reaction pathway, which has never been reported before," Zhang says. "I think this mechanism can also be generalized to other on-surface syntheses."
The team will next try other reaction excitation sources such as light to induce on-surface reactions and syntheses.
More information: Chi Zhang et al. Atomic-Scale Visualization of the Stepwise Metal-Mediated Dehalogenative Cycloaddition Reaction Pathways: Competition between Radicals and Organometallic Intermediates, Angewandte Chemie International Edition (2019). DOI: 10.1002/anie.201909111
Journal information: Angewandte Chemie International Edition
Provided by RIKEN