This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Molecular simulation AI tool reveals unresolved structure of transporter protein
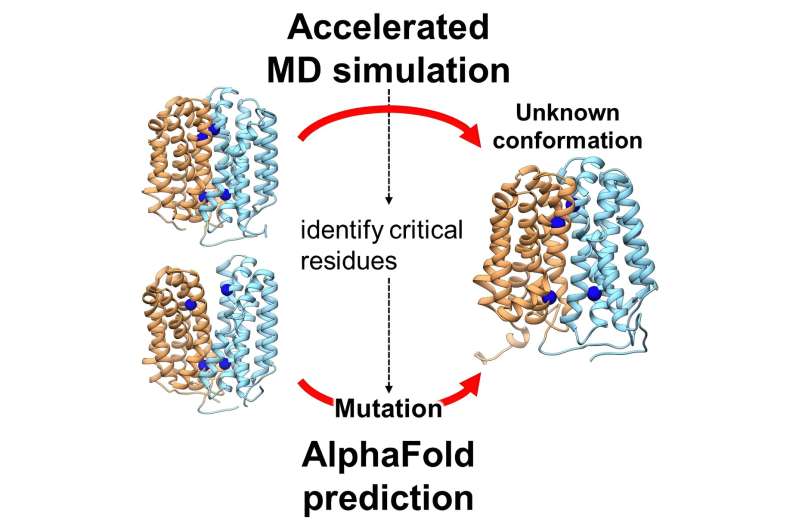
In a groundbreaking study, researchers have unveiled a previously unknown conformational state of a crucial transporter protein, OxlT, which plays a vital role in preventing kidney stone formation. This discovery, achieved through advanced computational methods, offers new insights into protein function and potential therapeutic targets.
Proteins are the building blocks of life, performing essential functions in every living organism. Transporter proteins, like OxlT, are particularly important as they carry vital substances across cell membranes. OxlT, found in the oxalate-degrading bacterium Oxalobacter formigenes, is instrumental in managing oxalate levels in the human body.
Excess oxalate can lead to kidney stones, a painful and prevalent health issue. Understanding OxlT's function is crucial, but until now, scientists lacked comprehensive knowledge of its various structural states, particularly the inward-open conformation, a critical part of its transport mechanism.
This study, led by Jun Ohnuki and his colleagues, utilized advanced computational techniques to simulate the OxlT protein's dynamics. They employed Gaussian accelerated molecular dynamics (GaMD) and AlphaFold2, a cutting-edge machine learning tool, to explore OxlT's structure and function. The paper, "Accelerated Molecular Dynamics and AlphaFold Uncover a Missing Conformational State of Transporter Protein OxlT," is published in The Journal of Physical Chemistry Letters.
The team successfully predicted the elusive inward-open conformation of OxlT, a significant step in understanding its complete functional cycle. This conformation revealed that OxlT prefers binding to formate rather than oxalate in this state, a crucial aspect of its role in oxalate management.
Furthermore, the research identified specific amino acid residues critical for this conformational transition, a finding that could have broader implications for understanding protein dynamics.
The implications of this research extend beyond a single protein. The methodology and insights obtained from this study provide a template for exploring other proteins' dynamics, particularly transporter proteins, which are often targets for therapeutic drugs.
Understanding these proteins at a detailed level can lead to the development of more effective treatments for a variety of conditions. Additionally, this research exemplifies the power of combining computational biology with machine learning, a rapidly evolving field that promises to unlock many of biology's most challenging mysteries.
By filling a crucial gap in our understanding of the OxlT protein, this study not only contributes to potential advancements in kidney stone prevention but also paves the way for future breakthroughs in biomedical research.
The research team includes Jun Ohnuki, Titouan Jaunet-Lahary and Kei-ichi Okazaki from the Research Center for Computational Science at Institute for Molecular Science (IMS), NINS. Completing the team is Atsuko Yamashita from the Graduate School of Medicine, Dentistry and Pharmaceutical Sciences at Okayama University.
More information: Jun Ohnuki et al, Accelerated Molecular Dynamics and AlphaFold Uncover a Missing Conformational State of Transporter Protein OxlT, The Journal of Physical Chemistry Letters (2024). DOI: 10.1021/acs.jpclett.3c03052
Journal information: Journal of Physical Chemistry Letters
Provided by National Institutes of Natural Sciences