This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
A new type of plant metalloreductase maintains root growth under low phosphorus
The release of low-molecular weight carboxylates, such as malate, is used by many plant species to mine poorly available phosphorus (P) from the soil. Malate can increase the availability of phosphate, the P form taken up by plants, by chelating trivalent aluminum or iron (Fe).
Work in the last years has demonstrated that the accumulation of Fe(III)-malate complexes in the apoplast of root tips attenuates root growth under low-P conditions because the now soluble Fe(III) (ferric Fe) participates in reactions leading to oxidative stress.
However, how root tips cope with this increased ferric Fe availability has remained elusive as all currently known ferric reductases, which are required for Fe uptake, are repressed in response to P deficiency or not expressed in root tips.
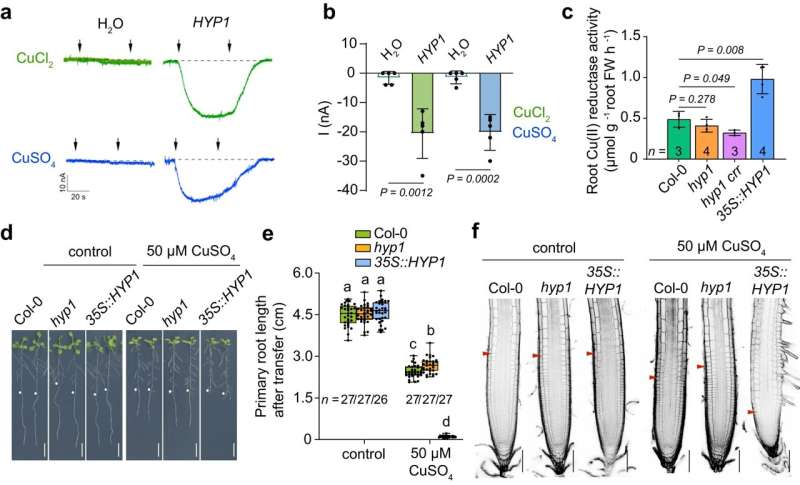
"By searching for other potential ferric reductases among P deficiency-induced genes, we found a previously uncharacterized gene encoding a domain predicted to participate in ferric reduction," explains Dr. Ricardo Giehl, co-leader of IPK's research group "Molecular Plant Nutrition." "When we disrupted this gene, Fe over accumulated in root tips, and root growth under low P was even more severely inhibited."
The gene was, therefore, named HYPERSENSITIVE TO LOW P1 (HYP1). HYP1 is a member of the CYBDOM family and possesses a cytochrome b561 domain, which is common in several ferric reductases active in animal cells, such as the duodenal cytochrome b (Dcytb) involved with dietary Fe uptake in humans.
"Although CYBDOMs are ubiquitous in several organisms and come typically as large families in plants, their function in any organism is still poorly understood," explains Rodolfo Maniero, first author of the study now published in Nature Communications.
Using AlphaFold-supported modeling and site-directed mutagenesis, the researchers found that HYP1 can coordinate three b-hemes, which are critical for the protein's activity. Electrophysiological characterization of HYP1 in oocytes revealed that the protein can transport electrons derived from ascorbate (vitamin C) across the plasma membrane. Acceptors of these electrons are Fe(III) and Cu(II) (cupric ion), indicating that the protein is a metalloreductase.
"Regarding the ferric reductase activity of HYP1, our results demonstrate that this function is critical to prevent malate-induced Fe overaccumulation in the apoplast and to maintain cell elongation and meristem integrity in root tips exposed to low P," explains Dr. Ricardo Giehl.
"Our results also suggested that an Fe-uptake mechanism is active in root tips, where the well-known ferric reductase FRO2 and the Fe(II) transporter IRT1 are not present," says the IPK scientist. "Our study not only shed light on the physiological roles of CYBDOMs but also identified a new target to improve root growth under limiting-P conditions."
More information: Rodolfo A. Maniero et al, Ferric reduction by a CYBDOM protein counteracts increased iron availability in root meristems induced by phosphorus deficiency, Nature Communications (2024). DOI: 10.1038/s41467-023-43912-w
Journal information: Nature Communications
Provided by Leibniz Institute of Plant Genetics and Crop Plant Research