This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Plants found to recruit distinct chemical activities of coumarins under different soil pHs
Plants have two main uptake mechanisms to obtain iron (Fe) from the soils. The type of strategy employed depends on the botanical classification of the plant. In the so-called strategy-I mechanism, plants must first reduce the trivalent iron (Fe3+) into bivalent iron (Fe2+). Only then can Fe2+ be taken up by roots as a free ion.
This strategy is used by non-Poaceae plants such as oilseed rape and the model plant Arabidopsis thaliana. Grasses, which belong to the Poaceae family, employ a so-called strategy II. These plants secrete chelating compounds, which can be reuptaken once chelated with Fe3+. Thus, no reduction step is required for Fe import into root cells.
"Interestingly, some Strategy-I plants also release metabolites into the soil through their roots when they suffer from Fe deficiency. Some of these are coumarins," explains IPK scientist Dr. Ricardo Giehl, co-head of the "Molecular Plant Nutrition" working group. However, the physiological role of these coumarins has not yet been sufficiently clarified.
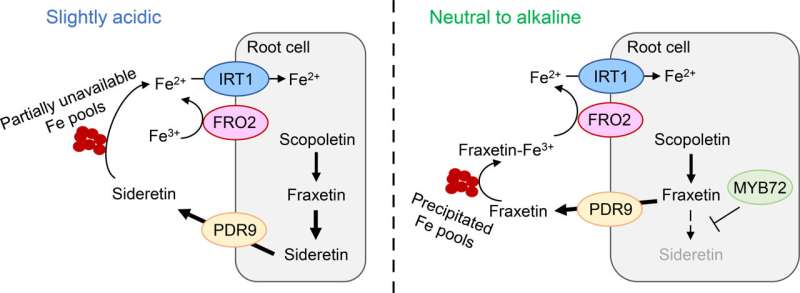
In their work, the researchers were able to show that the main function of two coumarins released in response to Fe deficiency is largely dependent on the external pH. Under slightly acidic conditions, coumarins, especially sideretin, help to sustain Fe3+ reduction.
Here, sideretin works together with the membrane-bound enzyme FERRIC REDUCTION OXIDASE 2 (FRO2) to efficiently Fe2+ uptake into the roots.
If the medium is alkaline, the biosynthesis of coumarins is shifted from sideretin to fraxetin, a response that the research team found to depend on the transcription factor MYB72. At alkaline pH, sideretin loses both its ability to reduce and even to solubilize Fe3+ from precipitated sources, while fraxetin retains a high Fe3+ mobilization capacity under such pH conditions. Therefore, rather than reducing Fe3+ directly, the main function of fraxetin is to provide soluble Fe(III)-chelates for FRO2-mediated reduction.
"Our study shows that by adjusting coumarin biosynthesis, plants recruit specific functions depending on the prevailing pH of the soil," says Dr. Ricardo Giehl. If the conditions are slightly acidic, plants favor the synthesis of the superior Fe3+ reductant sideretin, while at high pH, they direct synthesis towards fraxetin, which retains high Fe3+ mobilization capacity even under alkaline conditions.
With their work, the researchers provide valuable insights into environment-dependent fine regulation of metabolite biosynthesis and thus help to further understand how plants adapt to different pH conditions in the soil. The results open up new possibilities for the targeted improvement of plant productivity and plant health under variable soil conditions.
The study is published in The Plant Cell journal.
More information: Vanessa Paffrath et al, A major role of coumarin-dependent ferric iron reduction in strategy I-type iron acquisition in Arabidopsis, The Plant Cell (2023). DOI: 10.1093/plcell/koad279
Journal information: Plant Cell
Provided by Leibniz Institute of Plant Genetics and Crop Plant Research