This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Green methanol for the circular economy: Researchers develop new catalyst
Researchers hope to produce the raw material methanol at the edge of a field or on the farm using renewable energy. In addition to wind or sun, water and CO2 would be needed to produce the raw materials for the green methanol process: carbon monoxide (CO) and hydrogen (H2), which react catalytically to form methanol.
This is made possible by a new catalyst developed in Rostock. A process based on this completely dispenses with fossil raw materials. And it is highly selective, producing virtually no by-products.
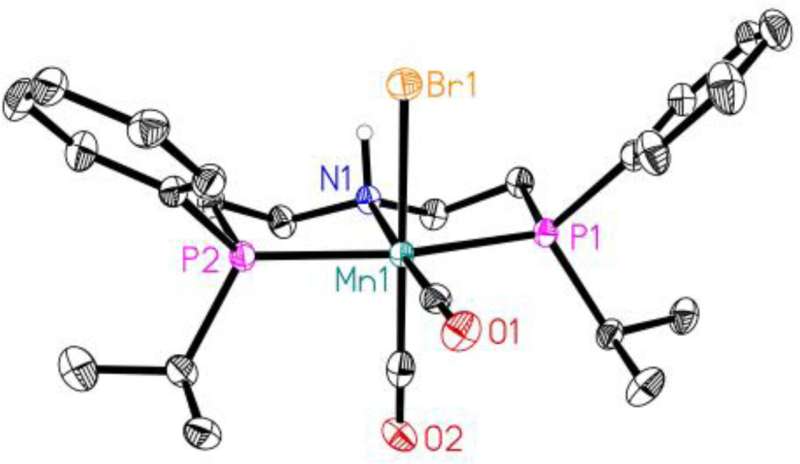
The catalyst is based on manganese, as Gordon Neitzel from the Leibniz Institute for Catalysis (LIKAT) explains, "The metal atom forms the catalytic center. It is fixed and protected by a kind of scaffold, the so-called ligand."
As part of his doctorate, Gordon Neitzel optimized the molecular structure of this ligand and put the finishing touches to the catalyst complex, so to speak. The results were published in the journal ChemCatChem.
Climate-neutral Management with E4MeWi
The work is part of the E4MeWi research network. The abbreviation stands for "Energie-Effiziente Erneuerbare Energien basierte Methanol-Wirtschaft" (Energy-Efficient Renewable Energy-based Methanol Economy). The project partners are CreativeQuantum GmbH in Berlin, Ineratec GmbH in Karlsruhe, Ruhr University Bochum and the Bitterfeld-Wolfen Chemical Park.
"A climate-neutral economy, as the Federal Republic of Germany is aiming for by 2045, also needs basic chemicals," Gordon Neitzel said.
Methanol is needed for plastics and resins, for example, which are used everywhere from the furniture to the automotive industry. Methanol production, currently 110 million metric tons per year worldwide, traditionally runs on natural gas—at high pressures of around 50 to 100 bar and temperatures between 200 and 300°C, depending on the process. With every ton of methanol, the huge plants emit one and a half tons of carbon dioxide. This has no future.
Reduced pressure and temperature requirements
The E4MeWi project aims to provide an alternative to the conventional process. Its core element is the catalyst, which allows H2 and CO to react in a dissolved state to produce methanol. The carbon monoxide is first extracted from CO2.
The manganese catalyst used for this was originally developed at LIKAT in the research group led by Dr. Kathrin Junge and Prof. Dr. Matthias Beller. It enables a completely new process that halves the pressure and temperature required for methanol production.
In addition, the process does not require fossil raw materials, which makes the catalyst a key element of a future CO2- and climate-neutral circular economy. Especially as methanol, produced in a green way, is also well suited as a chemical storage medium for hydrogen, one of the hopes of the energy transition.
Methanol plants in container size
The E4MeWi project participants envision a container-sized plant that uses local resources for sustainable value creation virtually at the edge of the field, on the farm or on the farmyard: Wind and solar energy, CO2 emissions from point sources and from biogas, plastic waste or wood waste. CO2 and water are initially combined to produce synthesis gas, a mixture of hydrogen and carbon monoxide, which is converted into methanol using the new catalyst.
Gordon Neitzel has significantly optimized the well-known manganese catalyst by developing new structures for the ligand that protectively surrounds the catalytically active center. "Without this shell, carbon monoxide would attack the manganese atom in the center of the catalyst and destroy the complex compound." This work has now doubled the reaction speed in methanol production.
This brings the project partners a good deal closer to an economically viable plant. After all, this is also part of the aim of such decentralized production: to establish a completely new market for the methanol trade and thus promote economic transformation processes.
More information: Gordon Neitzel et al, An Improved Manganese Pincer Catalyst for low Temperature Hydrogenation of Carbon Monoxide to Methanol, ChemCatChem (2023). DOI: 10.1002/cctc.202301053
Provided by Leibniz Institute for Catalysis