This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Unexpected discovery at the air-water interface for CO₂ reaction impacting geophysical and biological cycles
Ocean acidification, mammal respiration, and aerosol formation all depend on chemistry that occurs at air-water interfaces. In new research, scientists from the Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) have discovered which pathway carbon dioxide (CO2) molecules follow on their way from the atmosphere into water—and it's not the one that they expected.
The oceans absorb roughly 30% of all anthropogenic CO2 emissions. In water, the CO2 forms carbonic acid, changing the marine environment in ways that are harmful to some sea life. In our bodies, air that crosses the wet membranes lining our nasal tracts influences the pH of our blood.
But just how the local chemistry changes depends on how the dissolved CO2 separates into two different ions with different charges—doubly charged carbonate and singly charged bicarbonate—near the liquid surface. Berkeley Lab researchers now show an enhanced concentration of carbonate at air-water interfaces, where they expected to find more bicarbonate.
"The carbon cycle of Earth, as well as the respiration cycle of mammals, explicitly involves the dissolution of CO2 at the water surface and its transformation into bicarbonate and carbonate ions. Understanding reactions at the air–water interface will further illuminate these vitally important processes," said Jin Qian, a researcher who contributed the theoretical part of the work reported in the Journal of the American Chemical Society. Qian is a staff scientist in the Chemical Sciences Division at Berkeley Lab.
The chemical processes that occur at a liquid-air interface are often distinct from the same ones that occur in the corresponding bulk liquid. Textbook classical theory indicates that carbonate should remain in the bulk liquid, while bicarbonate should concentrate at the surface; but a detailed understanding of the pathways of the two ions has remained unclear. Because the surface of a solution comprises only a tiny fraction of its total volume, measuring ion concentrations there is difficult.
"Not only is the signal very weak, but it needs to be separated from the much larger bulk response of the system," explained Richard Saykally, a professor in UC Berkeley's Department of Chemistry, who led the work. Saykally is a retired senior faculty scientist in the Chemical Sciences Division at Berkeley Lab.
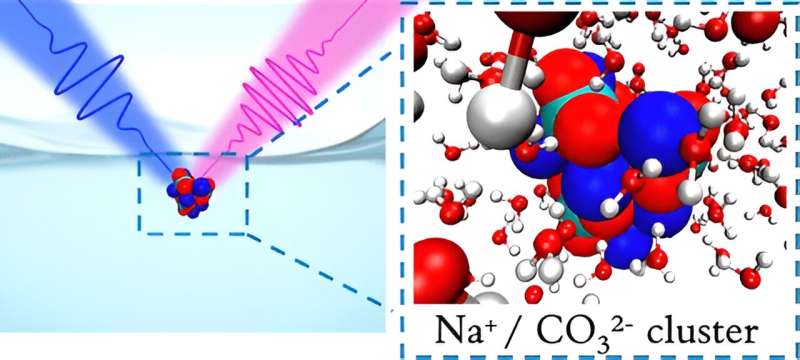
Saykally and his colleagues used tools specially designed to measure weak chemical signals at liquid surfaces. The technique, called deep UV second harmonic generation spectroscopy (DUV-SHG), directly probes ions at liquid interfaces.
"We can now measure the relative surface populations of carbonate and bicarbonate, as well as thermodynamic information regarding their surface affinity," said Shane Devlin, a postdoctoral researcher at Berkeley Lab and lead author on the study. The team found that carbonate exhibited a much stronger tendency to adhere to the surface than did bicarbonate.
To explain this highly unexpected behavior, the researchers turned to theoretical tools. Tod Pascal and his colleagues at UC San Diego ran computer simulations to understand how carbonate and bicarbonate ions form clusters, a process that was likely responsible for their different concentrations at the surface and in the bulk liquid.
They found that, while clustering was a favorable process for carbonate, it was not for bicarbonate. To further explain the spectroscopy observations, Qian and her group ran simulations using the Perlmutter system at the National Energy Research Scientific Computing Center (NERSC), a DOE user facility at Berkeley Lab. They developed a method that enabled calculations of the spectral fingerprints of carbonate and bicarbonate in a very large region at the liquid-air interface.
The simulations confirmed that carbonate does indeed exhibit a much stronger preference for the air-water interface. It resulted from the strong pairing of carbonate with sodium ions, which led to neutral clusters of particles that then became attracted to the surface.
"This is the first time that our computational method has been used in a realistic applicational setting, studying the air-liquid interface containing around a thousand atoms," says Qian.
While surprising, the measurement may have far-reaching implications. The ocean's surface is where air and water mix, leading to the formation of aerosol droplets, which play an essential role in global weather and atmospheric patterns.
As the level of atmospheric CO2 continues to rise, the ratio of carbonate and bicarbonate anions at the surface will likely shift, which in turn will influence the chemistry of marine aerosol droplets. Understanding the potential impact of increased carbonate concentrations in aerosols is important for scientists who are working to predict climate change.
In addition, bicarbonate is a relatively mild ion and can serve as a physiological buffer that helps our blood and tissues maintain proper chemistry and metabolic function. In contrast, carbonate is simply too strong to serve as a buffer. Understanding how these balances shift could be important for a thorough description of respiration in mammals.
"The interfacial behavior of these species and processes thus directly impacts both geophysical and biological cycles. The findings of this study will motivate future efforts aimed at ascertaining the consequences for the marine ecology," said Saykally.
More information: Shane W. Devlin et al, Agglomeration Drives the Reversed Fractionation of Aqueous Carbonate and Bicarbonate at the Air–Water Interface, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c05093
Journal information: Journal of the American Chemical Society
Provided by Lawrence Berkeley National Laboratory