This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Thianthrenium chemistry allows reactivity switch of a nucleophilic amino acid into a versatile intermediate
Chemical diversification of proteins is an important concept in the study of biological processes and the complex structures of the proteins themselves. Researchers from the Max Planck Society have now published their fascinating findings concerning an amino acid in Nature Chemistry.
Chemical diversification of proteins involves using quick and mild reactions that selectively target a specific amino acid and therefore a building block of proteins. Cysteine is a prominent example and can currently be modified in two ways. The first way requires the synthesis of electrophiles for each and every desired modification, e.g., a fluorescence probe that allows for following the molecule in very complex biological mixtures.
The second way turns cysteine itself into a chemical linchpin, which can then be diversified. Until now, this has been carried out in multistep syntheses. These methods have the drawback that the linchpin cannot be introduced in presence of external reagents required for its diversification. That is often accompanied by a limited choice of reagents for the functionalization as the linchpin needs to persist in solution during purification processes and has therefore an intrinsically decreased reactivity.
A new technique by the research group of Tobias Ritter, director at the Max-Planck-Institut für Kohlenforschung, is intriguing because it enables the introduction of a highly reactive intermediate in a one-pot process based on a single electrophile. Additionally, this method allows for a broad diversification of the new intermediate even in the presence of external reagents.
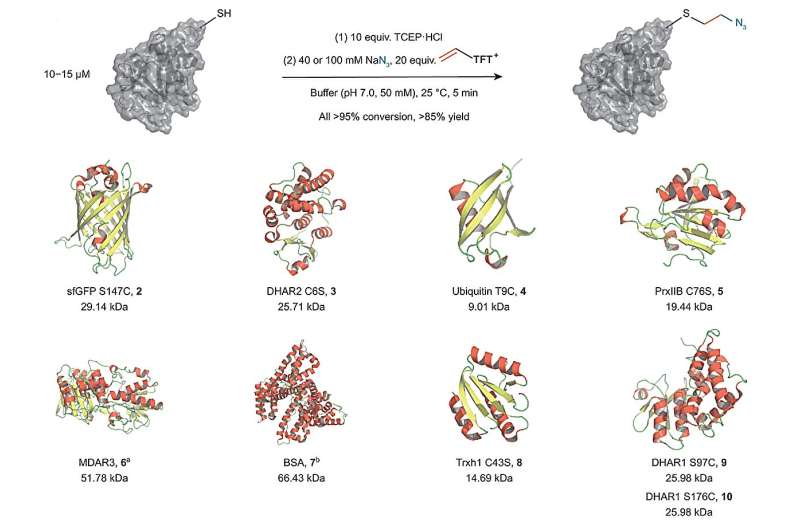
In their study, the Ritter group found a way to utilize vinyl thianthrenium salts to transform cysteine into a highly reactive episulfonium electrophile in situ. That approach allows to connect cysteine with various other external nucleophiles in a single one-pot process without the need for additional steps. The method enables the scientists to link different biorelevant functional groups to proteins using a short and stable ethylene linkage very close to the protein's surface. Hence, providing a new and attractive way to add labels or functionalities that alter the chemical environment of a protein.
When there are no external nucleophiles added, other amino acids can react with the episulfonium intermediate in an intramolecular reaction. That reactivity allows for protein-protein ligation and macrocyclization of linear peptides. While the first approach allows to study protein complexes and their often altered biological activity, the second approach makes the peptides more stable towards biological degradation if used, for example, as a drug.
Additionally, the synthesis of vinyl thianthrenium salts from ethylene gas allowed the Ritter group to synthesize reagents with a different composition of isotopes. Those isotope-labeled compounds possess the same reactivity as the non-labeled derivatives but slightly differ in their molecular weight. Hence, they can be utilized in state-of-the-art mass spectrometry proteomics research to extract quantitative information from whole cellular systems. Overall, the method using vinyl thianthrenium salts is showcased as a useful and broadly applicable tool in the field of chemical biology.
More information: Philipp Hartmann et al, Chemoselective umpolung of thiols to episulfoniums for cysteine bioconjugation, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01388-7
Journal information: Nature Chemistry
Provided by Max Planck Society