This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop irreversible inhibitor to address proteins that have acquired drug-resistant mutations
The idea of irreversible inhibitors adhering permanently to a target protein has gained increasing attention for application in potential drug development. However, one of many hurdles is the possibility of protein mutations making otherwise effective drugs pharmacologically inactive.
Current covalent inhibitors have reactive groups known to induce a single reaction in target proteins, irreversibly turning them off. Sometimes, however, mutations can occur more easily with specific amino acids, interfering with this deactivation.
Now, a team of researchers at Kyoto University has developed a new reactant demonstrating efficacy on proteins that have acquired drug-resistant mutations.
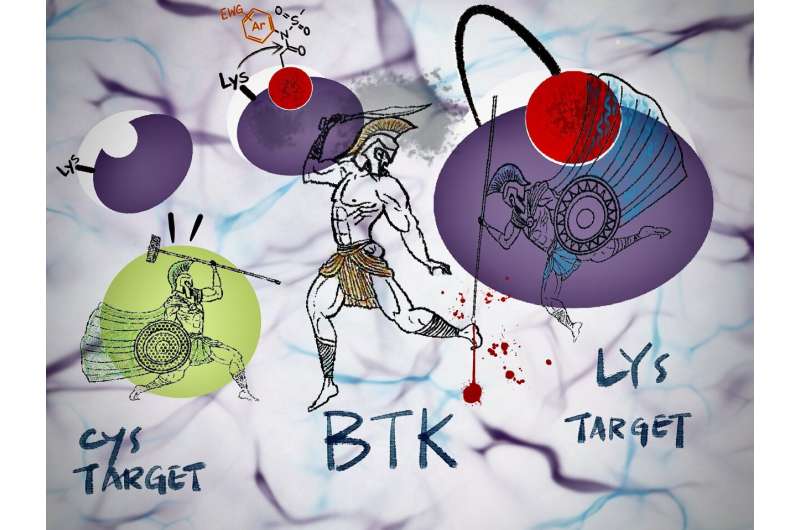
"In Bruton-type tyrosine kinase (BTK), an important drug target, a mutation involving amino acids cysteine to serine—called C481S—is known, but we have not yet seen any for our lysine target," remarks Tomonori Tamura of the Graduate School of Engineering.
"Still, it is significant that our irreversible inhibitor can at least address the C481S problem," Tamura adds.
Conventional irreversible inhibitors used in clinical practice react only with protein cysteine residues.
In addition, cysteine—the most reactive among the 20 amino acids—is not abundant at reaction or binding sites. This amino acid can be mutated into a different amino acid, making the cysteine-targeting irreversible inhibitors ineffective against drug-resistant proteins.
In contrast, N-acyl-N-arylsulfonamide, or ArNASA, can react with lysine residues and is highly stable in serum-containing media and other physiological environments.
"Utilizing this reaction property, we developed the first irreversible inhibitor of BTK, which has drug-resistant mutations," adds Tamura.
The Tamura team's hunt for useful reactive groups may come to fruition with ArNASA. Importantly, its electrophiles remove limiting factors by minimizing hydrolytic inactivation and unintended reactions with off-target proteins.
Once the target engages with the irreversible inhibitor, the reactive group chemically reacts with the protein's amino acids to form a covalent bond. An unrelenting binding site results, irreversibly inhibiting protein activity.
Tamura's team improved upon an earlier NASA group—similar in efficacy as ArNASA but ineffective in serum-containing media—by synthesizing the new reactive group using aromatic amines as starting materials. The researchers applied the ArNASA group to BTK, an important therapeutic target for blood cancers such as chronic lymphocytic leukemia.
"Our study will extend beyond cell-based research to in vivo, paving the way for developing drugs with diverse reactant groups acting on specific amino acids," concludes Tamura.
The research is published in the Journal of the American Chemical Society.
More information: Masaharu Kawano et al, Lysine-Reactive N-Acyl-N-aryl Sulfonamide Warheads: Improved Reaction Properties and Application in the Covalent Inhibition of an Ibrutinib-Resistant BTK Mutant, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c08740
Journal information: Journal of the American Chemical Society
Provided by Kyoto University